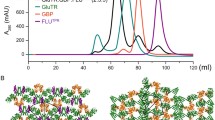
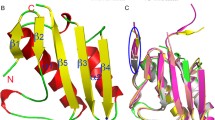
Abstract
Glutamyl-tRNA reductase (GluTR) catalyzes the first step of tetrapyrrole biosynthesis in plants, archaea and most bacteria. The catalytic mechanism of the enzyme was elucidated both by biochemical data and the determination of the high-resolution crystal structure of the enzyme from the archaeon Methanopyrus kandleri in complex with a competitive inhibitor. The dimeric enzyme has an unusual V-shaped architecture where each monomer consists of three domains linked by a long `spinal' α-helix. The central catalytic domain specifically recognizes the glutamate moiety of the substrate. It bears a conserved cysteine poised to nucleophilically attack the activated aminoacyl bond of glutamyl-tRNA. Subsequently, the thioester intermediate is reduced to the product glutamate-1-semialdehyde via hydride transfer from NADPH supplied by the second domain. A structure-based sequence alignment indicates that catalytically essential amino acids are conserved throughout all GluTRs. Thus the catalytic mechanism derived for M. kandleri is common to all including plant GluTRs. Mutations described to influence the catalytic efficiency of the barley enzyme can therefore be explained.
Similar content being viewed by others
References
Avissar YJ, Ormerod JG and Beale SI (1989) Distribution of delta-aminolevulinic acid biosynthetic pathways among phototrophic bacterial groups. Arch Microbiol 151: 513–519
Beale SI and Castelfranco PA (1973) C incorporation from exogenous compounds into ?-aminolevulinic acid by greening cucumber cotyledons. Biochem Biophys Res Commun 52: 143–149
Carugo O and Argos P (1997) NADP-dependent enzymes. I. Conserved stereochemistry of cofactor binding. Proteins Struct Genet 28: 10–28
Cavarelli J and Moras D (1993) Recognition of tRNAs by aminoacyl-tRNA synthetases. FASEB J 7: 79–86.
Chen MW, Jahn D, O'Neill GP and Söll D (1990) Purification of the glutamyl-tRNA reductase from Chlamydomonas reinhardtii involved in delta-aminolevulinic acid formation during chlorophyll biosynthesis. J Biol Chem 265: 4058–4063
Contestabile R, Angelaccio S, Maytum R, Bossa F and John RA (2000) The contribution of a conformationally mobile, active site loop to the reaction catalyzed by glutamate semialdehyde aminomutase. J Biol Chem 275:, 3879–3886
Eiler S, Dock-Bregeon A, Moulinier L, Thierry JC and Moras D (1999) Synthesis of aspartyl-tRNA(Asp) in Escherichia coli-a snapshot of the second step. EMBO J 18: 6532–6541
Ferreira GC (1995) Heme biosynthesis: biochemistry, molecular biology, and relationship to disease. J Bioenerg Biomembr 27: 147–50
Gouet P, Courcelle E, Stuart DI and Metoz F (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15: 305–308
Grimm B (1990) Primary structure of a key enzyme in plant tetrapyrrole synthesis: glutamate 1-semialdehyde aminotransferase. Proc Natl Acad Sci USA 87: 4169–4173
Hennig M, Grimm B, Contestabile R, John RA and Jansonius JN (1997) Crystal structure of glutamate-1-semialdehyde aminomutase: an ?2-dimeric vitamin B6-dependent enzyme with asymmetry in structure and active site reactivity. Proc Natl Acad Sci USA 94: 4866–4871
Ilag LL and Jahn D (1992) Activity and spectroscopic properties of the Escherichia coli glutamate 1-semialdehyde aminotransferase and the putative active site mutant K265R. Biochemistry 31: 7143–7151
Jahn D, Chen MW and Söll D (1991) Purification and functional characterization of glutamate-1-semialdehyde aminotransferase from Chlamydomonas reinhardtii. J Biol Chem 266: 161–167
Jahn D, Verkamp E and Söll D (1992) Glutamyl-transfer RNA: a precursor of heme and chlorophyll biosynthesis. Trends Biochem Sci 17: 215–218
Kabsch W and Sander C (1983). Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22: 2577–2637
Kikuchi G, Kumar A, Talmage P and Shemin D (1958) The enzymatic synthesis of ?-aminolevulinic acid. J Biol Chem 233: 1214–1219
Kraulis PJ (1991) MOLSCRIPT: a program to produce both detailed and schematic plots or protein structures. J Appl Crystallogr 24: 946–950
Mayer SM, Beale SI and Weinstein JD. (1987) Enzymatic conversion of glutamate to delta-aminolevulinic acid in soluble extracts of Euglena gracilis. J Biol Chem 262: 12541–12549
Merrit EA and Murphy MEP (1994) Raster3D version 2.0: a program for photorealistic molecular graphics. Acta Crystallogr D50: 869–873
Moser J, Lorenz S, Hubschwerlen C, Rompf A and Jahn D (1999) Methanopyrus kandleri glutamyl-tRNA-reductase. J Biol Chem 274: 30679–30685
Moser J, Schubert W-D, Beier V, Jahn D and Heinz DW (2001) V-shaped structure of glutamyl-tRNA-reductase, the first enzyme of tRNA-dependent tetrapyrrole biosynthesis. EMBO J 20: 6583–6590
Neuberger A (1968) Biochemical basis and regulatory mechanisms of porphyrin synthesis. Proc R Soc Med 61: 191–193
Schön A, Krupp G, Gough S, Berry-Lowe S, Kannangara CG and Söll D.(1986) The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature 322: 281–284
Sekine S, Nureki O, Shimada A, Vassylyev DG and Yokoyama S (2001) Structural basis for anticodon recognition by discriminating glutamyl-tRNA synthetase. Nat Struct Biol 8: 189–191
Smith MA, Kannangara CG and Grimm B (1992) Glutamate 1-semialdehyde aminotransferase: anomalous enantiomeric reaction and enzyme mechanism. Biochemistry 31: 11249–11254
Thompson JD, Higgins DG and Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680
Verkamp E, Jahn M, Jahn D, Kumar AM and Söll D (1992) Glutamyl-tRNA reductase from Escherichia coli and Synechocystis 6803. Gene structure and expression. J Biol Chem 267: 8275–8280
Vothknecht UC, Kannangara CG and von Wettstein D (1996) Expression of catalytically active barley glutamyl tRNAGlu reductase in Escherichia coli as a fusion protein with glutathione S-transferase. Proc Natl Acad Sci USA 93: 9287–9291
Vothknecht UC, Kannangara CG and von Wettstein D (1998) Barley glutamyl tRNAGlu reductase: mutations affecting haem inhibition and enzyme activity. Phytochemistry 47: 513–519
Willows RD, Kannangara CG and Pontoppidan B (1995) Nucleotides of tRNA (Glu) involved in recognition by barley chloroplast glutamyl-tRNA synthetase and glutamyl-tRNA-reductase. Biochim Biophys Acta 1263: 228–234
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schubert, WD., Moser, J., Schauer, S. et al. Structure and function of glutamyl-tRNA reductase, the first enzyme of tetrapyrrole biosynthesis in plants and prokaryotes. Photosynthesis Research 74, 205–215 (2002). https://doi.org/10.1023/A:1020963711861
Issue Date:
DOI: https://doi.org/10.1023/A:1020963711861