Abstract
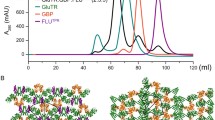
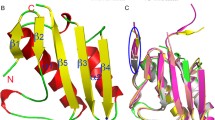
In higher plants, the tetrapyrrole biosynthesis pathway starts from the reaction catalyzed by the rate-limiting enzyme, glutamyl-tRNAGlu reductase (GTR). In Arabidopsis thaliana, GTR is controlled by post-transcriptional regulators such as GTR binding protein (GBP), which stimulates AtGTR activity. The NADPH-binding domain of AtGTR undergoes a substantial movement upon GBP binding. Here, we report the crystal structure of AtGTR-NADPH-GBP ternary complex. NADPH binding causes slight structural changes compared with the AtGTR-GBP binary complex, and possibly take a part of the space needed by the substrate glutamyl-tRNAGlu. The highly reactive sulfhydryl group of the active-site residue Cys144 shows an obvious rotation, which may facilitate the hydride transfer from NADPH to the thioester intermediate to form glutamate-1-semialdehyde. Furthermore, Lys271, Lys274, Ser275, Asn278, and Gln282 of GBP participate in the interaction between AtGTR and GBP, and the stimulating effect of GBP decreased when all of these residues were mutated to Ala. When the Cys144 of AtGTR was mutated to Ser, AtGTR activity could not be detected even in the presence of GBP.








Similar content being viewed by others
Abbreviations
- ALA:
-
5-Aminolevulinic acid
- GBP:
-
Glutamyl-tRNAGlu reductase binding protein
- GSA:
-
Glutamate-1-semialdehyde
- GSAT:
-
Glutamate-1-semialdehyde 2,1-aminomutase
- GTR:
-
Glutamyl-tRNAGlu reductase
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- RMSD:
-
Root-mean-square deviation
- SEM:
-
Standard error of mean
- TPR:
-
Tetratricopeptide-repeat
References
Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD (2012) Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr Sect D 68:352–367. https://doi.org/10.1107/S0907444912001308
Apitz J, Nishimura K, Schmied J, Wolf A, Hedtke B, van Wijk KJ, Grimm B (2016) Posttranslational control of ALA synthesis includes GluTR degradation by Clp protease and stabilization by GluTR-binding protein. Plant Physiol 170:2040–2051. https://doi.org/10.1104/pp.15.01945
Avissar YJ, Moberg PA (1995) The common origins of the pigments of life-early steps of chlorophyll biosynthesis. Photosynth Res 44:221–242. https://doi.org/10.1007/BF00048596
Czarnecki O, Hedtke B, Melzer M, Rothbart M, Richter A, Schroter Y, Pfannschmidt T, Grimm B (2011) An Arabidopsis GluTR binding protein mediates spatial separation of 5-aminolevulinic acid synthesis in chloroplasts. Plant Cell 23:4476–4491. https://doi.org/10.1105/tpc.111.086421
Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr Sect D 66:486–501. https://doi.org/10.1107/S0907444910007493
Fang Y, Zhao S, Zhang F, Zhao A, Zhang W, Zhang M, Liu L (2016) The Arabidopsis glutamyl-tRNA reductase (GluTR) forms a ternary complex with FLU and GluTR-binding protein. Sci Rep 6:19756. https://doi.org/10.1038/srep19756
Gargouri M, Gallois B, Chaudiere J (2009) Binding-equilibrium and kinetic studies of anthocyanidin reductase from Vitis vinifera. Arch Biochem Biophys 491:61–68. https://doi.org/10.1016/j.abb.2009.09.010
Goncalves S, Miller SP, Carrondo MA, Dean AM, Matias PM (2012) Induced fit and the catalytic mechanism of isocitrate dehydrogenase. Biochemistry 51:7098–7115. https://doi.org/10.1021/bi300483w
Hearn WR, Wildfeuer ME (1961) A synthesis of 5-aminolevulinic acid analogous to its biosynthesis. Anal Biochem 2:140–146
Jung HS, Okegawa Y, Shih PM, Kellogg E, Abdel-Ghany SE, Pilon M, Sjolander K, Shikanai T, Niyogi KK (2010) Arabidopsis thaliana PGR7 encodes a conserved chloroplast protein that is necessary for efficient photosynthetic electron transport. PLoS One 5:e11688. https://doi.org/10.1371/journal.pone.0011688
Kauss D, Bischof S, Steiner S, Apel K, Meskauskiene R (2012) FLU, a negative feedback regulator of tetrapyrrole biosynthesis, is physically linked to the final steps of the Mg(++)-branch of this pathway. FEBS Lett 586:211–216. https://doi.org/10.1016/j.febslet.2011.12.029
Kleiger G, Eisenberg D (2002) GXXXG and GXXXA motifs stabilize FAD and NAD(P)-binding Rossmann folds through C(alpha)-H… O hydrogen bonds and van der waals interactions. J Mol Biol 323:69–76
Kleiger G, Grothe R, Mallick P, Eisenberg D (2002) GXXXG and AXXXA: common alpha-helical interaction motifs in proteins, particularly in extremophiles. Biochemistry 41:5990–5997
Laskowski RA, Macarthur MW, Moss DS, Thornton JM (1993) Procheck—a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Lobley CM, Ciulli A, Whitney HM, Williams G, Smith AG, Abell C, Blundell TL (2005) The crystal structure of Escherichia coli ketopantoate reductase with NADP + bound. Biochemistry 44:8930–8939. https://doi.org/10.1021/bi0502036
Luer C, Schauer S, Mobius K, Schulze J, Schubert WD, Heinz DW, Jahn D, Moser J (2005) Complex formation between glutamyl-tRNA reductase and glutamate-1-semialdehyde 2,1-aminomutase in Escherichia coli during the initial reactions of porphyrin biosynthesis. J Biol Chem 280:18568–18572. https://doi.org/10.1074/jbc.M500440200
Masuda T, Fujita Y (2008) Regulation and evolution of chlorophyll metabolism. Photochem Photobiol Sci 7:1131–1149. https://doi.org/10.1039/b807210h
McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ (2007) Phaser crystallographic software. J Appl Crystallogr 40:658–674. https://doi.org/10.1107/S0021889807021206
Meskauskiene R, Apel K (2002) Interaction of FLU, a negative regulator of tetrapyrrole biosynthesis, with the glutamyl-tRNA reductase requires the tetratricopeptide repeat domain of FLU. FEBS Lett 532:27–30
Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K (2001) FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 98:12826–12831. https://doi.org/10.1073/pnas.221252798
Moser J, Lorenz S, Hubschwerlen C, Rompf A, Jahn D (1999) Methanopyrus kandleri glutamyl-tRNA reductase. J Biol Chem 274:30679–30685
Moser J, Schubert WD, Beier V, Bringemeier I, Jahn D, Heinz DW (2001) V-shaped structure of glutamyl-tRNA reductase, the first enzyme of tRNA-dependent tetrapyrrole biosynthesis. EMBO J 20:6583–6590. https://doi.org/10.1093/emboj/20.23.6583
Nogaj LA, Beale SI (2005) Physical and kinetic interactions between glutamyl-tRNA reductase and glutamate-1-semialdehyde aminotransferase of Chlamydomonas reinhardtii. J Biol Chem 280:24301–24307. https://doi.org/10.1074/jbc.M502483200
Otwinowski Z, Borek D, Majewski W, Minor W (2003) Multiparametric scaling of diffraction intensities. Acta Crystallogr Sect A 59:228–234
Schauer S, Chaturvedi S, Randau L, Moser J, Kitabatake M, Lorenz S, Verkamp E, Schubert WD, Nakayashiki T, Murai M, Wall K, Thomann HU, Heinz DW, Inokuchi H, Soll D, Jahn D (2002) Escherichia coli glutamyl-tRNA reductase. Trapping the thioester intermediate. J Biol Chem 277:48657–48663. https://doi.org/10.1074/jbc.M206924200
Schubert WD, Moser J, Schauer S, Heinz DW, Jahn D (2002) Structure and function of glutamyl-tRNA reductase, the first enzyme of tetrapyrrole biosynthesis in plants and prokaryotes. Photosynth Res 74:205–215. https://doi.org/10.1023/A:1020963711861
Seo KH, Zhuang N, Chen C, Song JY, Kang HL, Rhee KH, Lee KH (2012) Unusual NADPH conformation in the crystal structure of a cinnamyl alcohol dehydrogenase from Helicobacter pylori in complex with NADP(H) and substrate docking analysis. FEBS Lett 586:337–343. https://doi.org/10.1016/j.febslet.2012.01.020
Srivastava A, Beale SI (2005) Glutamyl-tRNA reductase of Chlorobium vibrioforme is a dissociable homodimer that contains one tightly bound heme per subunit. J Bacteriol 187:4444–4450. https://doi.org/10.1128/JB.187.13.4444-4450.2005
Tanaka R, Tanaka A (2007) Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58:321–346. https://doi.org/10.1146/annurev.arplant.57.032905.105448
Tomita T, Fushinobu S, Kuzuyama T, Nishiyama M (2005) Crystal structure of NAD-dependent malate dehydrogenase complexed with NADP(H). Biochem Biophys Res Commun 334:613–618. https://doi.org/10.1016/j.bbrc.2005.06.133
Vothknecht UC, Kannangara CG, von Wettstein D (1996) Expression of catalytically active barley glutamyl tRNAGlu reductase in Escherichia coli as a fusion protein with glutathione S-transferase. Proc Natl Acad Sci USA 93:9287–9291
Waters MT, Langdale JA (2009) The making of a chloroplast. EMBO J 28:2861–2873. https://doi.org/10.1038/emboj.2009.264
Zhang M, Zhang F, Fang Y, Chen X, Chen Y, Zhang W, Dai HE, Lin R, Liu L (2015) The non-canonical tetratricopeptide repeat (TPR) domain of fluorescent (FLU) mediates complex formation with glutamyl-tRNA reductase. J Biol Chem 290:17559–17565. https://doi.org/10.1074/jbc.M115.662981
Zhao A, Fang Y, Chen X, Zhao S, Dong W, Lin Y, Gong W, Liu L (2014) Crystal structure of Arabidopsis glutamyl-tRNA reductase in complex with its stimulator protein. Proc Natl Acad Sci USA 111:6630–6635. https://doi.org/10.1073/pnas.1400166111
Acknowledgements
We thank Dr. Liu Lin from the Institute of Botany of the Chinese Academy of Sciences for her support of this work. This work was financially supported by the National Natural Science Foundation of China (31500243 and 31501338) and the National Basic Research Program of China (Z109021566).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, A., Han, F. Crystal structure of Arabidopsis thaliana glutamyl-tRNAGlu reductase in complex with NADPH and glutamyl-tRNAGlu reductase binding protein. Photosynth Res 137, 443–452 (2018). https://doi.org/10.1007/s11120-018-0518-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-018-0518-8