Abstract
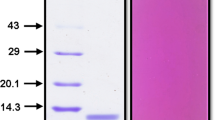
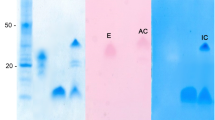
A proteinase inhibitor resembling Bowman-Birk family inhibitors has been purified from the seeds of cultivar HA-3 of Dolichos lablab perpureus L. The protein was apparently homogeneous as judged by SDS–PAGE, PAGE, IEF, and immunodiffusion. The inhibitor had 12 mole% 1/2-cystine and a few aromatic amino acids, and lacks tryptophan. Field bean proteinase inhibitor (FBPI) exhibited a pI of 4.3 and an M r of 18,500 Da. CD spectral studies showed random coiled secondary structure. Conformational changes were detected in the FBPI–trypsin/chymotrypsin complexes by difference spectral studies. Apparent K a values of complexes of inhibitor with trypsin and chymotrypsin were 2.1 × 107 M−1 and 3.1 × 107 M−1, respectively. The binary and ternary complexes of FBPI with trypsin and chymotrypsin have been isolated indicating 1:1 stoichiometry with independent sites for cognate enzymes. Amino acid modification studies showed lysine and tyrosine at the reactive sites of FBPI for trypsin and chymotrypsin, respectively.
Similar content being viewed by others
REFERENCES
Banerji, A. P., and Sohani, K. (1969). Enzymologia 36, 137–152.
Broadway, R. M. (1993), Phytochemistry 33, 21–27.
Davis, B. J. (1964). Ann. N. Y. Acad. Sci. 121, 404–427.
Feinstein, G., and Feeney, R. E. (1966). J. Biol. Chem. 241, 5183–5189.
Grassmann, M., Thommes, P., Weiser, T., and Hubscher, U. (1990). FASEB J. 4, 2528–2532.
Grob, D. (1950). J. Gen. Physiol. 33, 103–124.
Habeeb, A. F. S. A. (1972). Meth. Enzymol. 25, 457–464.
Haldar, U. C., Saha, S. K., Beavis, R. C., and Sinha, N. K. (1996). J. Protein Chem. 15, 177–185.
Haynes, R., and Feeney, R. E. (1967). J. Biol. Chem. 242, 5378–5385.
Hilder, V. A., Gatehouse, A. M. R., Sheerman, S. E., Barker, R. F., and Boulter, D. (1987). Nature 330, 160–163.
Ikenaka, T., and Norioka, S. (1986). In Proteinase Inhibitors (Barrett, A. J., and Salvesen, G., eds.), Elsevier, Amsterdam, pp. 361–374.
Kakade, M. L., Simson, N., and Liener, I. E. (1969). Ceral Chem. 46, 518–526.
Kakade, M. L., Swenson, D. H., and Liener, I. E. (1970). Anal. Biochem. 33, 255–258.
Kennady, A. R. (1994). Cancer Res. (Suppl.) 54, 1999s-2005s.
Laskowski, M., Jr. (1986). In Nutritional and Toxicological Significance of Enzyme Inhibitors in Foods (Friedman, M., ed.), Plenum Press, New York, pp. 1–17.
Laskowski, M., Jr., and Kato, L. (1980). Annu. Rev. Biochem. 49, 593–626.
Liu, W. H., Feinstein, G., Osuga, D. T., Haynes, R., and Feeney, R. E. (1968). Biochemistry 7, 2886–2892.
Lowery, O. H., Rosebrough, N. J., Fan, A. L., and Randal, R. J. (1951). J. Biol. Chem. 193, 265–275.
Manjunatha, N. H., Veerabhadrappa, P. S., and Virupaksha, T. K. (1983). Phytochemistry 22, 2349–2357.
Moosor, G., Skupin, J., and Romanowska, B. (1984). Nahrung 28, 93–112.
Norton, G. (1991). In Toxic Substances in Crop Plants (D'Mello, J. P. F., Duffus, C. M., and Duffus, J. H., eds.), Royal Society of Chemistry, London, pp. 68–106.
Oswole, O., John, A., Tamkari, N., and Ouwaifo, A. (1992). Biochem. Pharmacol. 43, 1880–1882.
Ouchterloney, Ö. (1967). In Handbook of Experimental Immunology (Weir, D. M., ed.), Blackwell, Oxford, pp. 655–706.
Ramasarma, P. R., and Rajagopal Rao, D. (1991). Ind. J. Biochem. Biophys. 28, 418–424.
Rayas-Duarte, P., Bergeron, D., and Neilsen, S. (1992). J. Agric. Food Chem. 40, 32–42.
Richardson, M. (1991). Meth. Plant Biochem. 5, 260–305.
Riordan, J. F., and Valle, B. L. (1972). Meth. Enzymol. 25, 500–506.
Ryan, C. A. (1990). Annu. Rev. Phytopathol. 28, 425–449.
Sammons, D. W., Adam, L. D., and Nishigawa, E. E. (1981). Electrophoresis 2, 135–140.
Schwert, G. W., and Takenaka, Y. (1955). Biochem. Biophys. Acta 16, 570–575.
Shulmina, A., Dronova, L. L. A., Shubin, V. V., Levenskaya, O. A., and Muslov, V. V. (1985). Biokhimiya 50, 1151–1154.
Spackman, D. H., Stein, W. H., and Moore, S. (1958). Anal. Chem. 30, 1190–1206.
Weber, K., and Osborn, M. (1969). J. Biol. Chem. 244, 4406–4412.
Weder, J. K. P. (1985). Qual. Plant Food Hum. Nutr. 35, 183–194.
Xavier-Filho, J., and Moreira, R. D. A. (1978). Anal. Biochem. 84, 296–303.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Devaraj, V.R., Manjunatha, N.H. Purification and Characterization of a Proteinase Inhibitor from Field Bean, Dolichos lablab perpureus L.. J Protein Chem 18, 47–54 (1999). https://doi.org/10.1023/A:1020695315964
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1020695315964