Abstract
Objective: Although improvementshave been made in the management ofpatients with advanced ovarian cancer,long-term survivors are still uncommon. Gemcitabine and prolonged oral etoposidehave shown reproducible single-agentactivity in patients withplatinum/paclitaxel-resistant ovariancancer. This, combined with preclinicalsynergism, prompted the GynecologicOncology Group to determine the maximumtolerated dose (MTD) of this combination.
Methods: Eligible patients hadrecurrent epithelial ovarian cancer,primary papillary peritoneal, or fallopiantube carcinoma. All had received priorplatinum/paclitaxel-based chemotherapy andhad adequate hepatic, renal and bone marrowfunction. Oral etoposide was administeredat 50 mg/m2 for ten days, with threeproposed dose levels for gemcitabine ondays 1 and 8: 400, 550 and 700 mg/m2. Cycles were to be repeated every 28 days. Three patients were to enter at each doselevel.
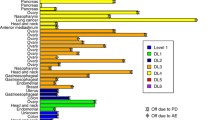
Results: Patients were enrolled onlyto dose level 1 as this dose exceeded MTD. Of six patients initially enrolled, one wasremoved after three days with fever,ascites and decreased albumin believed notto be treatment related. Five patientswere evaluable for toxicity and response. One of the first three patients developeddose limiting toxicity (DLT) manifested asgrade 4 neutropenia. A second DLT(neutropenic fever and thrombocytopeniaassociated with bleeding) occurred amongthe next three patients; therefore, MTD wasreached at dose level 1. Grade 4toxicities included episodes of neutropenia(4) and thrombocytopenia (3). No objectiveresponse was observed.
Conclusions: Oral etoposide andgemcitabine at this dose and schedule wasassociated with substantial toxicity inthis population. Patients who are previously treated withplatinum/paclitaxel-based chemotherapy maybe at particular risk for toxicity.
Similar content being viewed by others
References
Omura G, Blessing JA, Ehrlich CE, Miller A, Yordan E, Creasman WT, Homesley HD: A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma: a Gynecology Oncology Group Study. Cancer 57:1725–1730, 1986
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge E, Look KY, Clarke-Pearson DL, Davidson M: Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 334:1–6, 1996
Ozols RF, Bundy BN, Fowler J, Clarke-Pearson D, Mannel R, Hartenbach EM, Baergen R: Randomized phase III study of cisplatin (cis)/paclitaxel (pac) versus carboplatin (carbo)/pac in optimal stage III epithelial ovarian cancer (oc): a Gynecologic Oncology Group trial. Proc ASCO 18:356a, 1999 (abstract)
Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, Jones W, Almadrones L, Lewis JL: Second line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 9:389–393, 1991
Bookman MA, Malmstrom H, Bolis G, Gordon A, Lissoni A, Krebs JB, Fields SZ: Topotecan for the treatment of advanced epithelial ovarian cancer: an open-label phase II study in patients treated after prior chemotherapy containing cisplatin or carboplatin and paclitaxel. J Clin Oncol 16:3345–3352, 1998
Gordon AN, Granai CO, Rose PG, Hainsworth J, Lopez A, Weissman C, Rosales R, Sharpington T: Phase II study of liposomal doxorubicin in platinum-and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol 18:3093–3100, 2000
Rose PG, Blessing JA, Mayer AR, Homesley HD: Prolonged oral etoposide as second line therapy for platinum/paclitaxel-resistant and platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 16:405–410, 1998
Shapiro JD, Millward MJ, Rischin D, Michael M, Walcher V, Francis PA, Toner GC: Activity of gemcitabine in patients with advanced ovarian cancer: responses seen following platinum and paclitaxel. Gynecol Oncol 63:89–93, 1996
Kavanaugh JJ, Winn R, Steger M, Nelson-Taylor T, Edwards K, Rodgers R, Borst J, Kudelka AP, Hu W, Vershraegen CF: Docetaxel for patients with ovarian cancer refractory to paclitaxel, an update. Proc ASCO 18:368a, 1999 (abstract)
Hande KR: Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer 34:1514–1521, 1998
Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W: Action of 2′2′ difluorodeoxycytidine on DNA synthesis. Cancer Res 51:6110–6117, 1991
Nakamura H, Yamaji Y, Fujita J, Takahara J: Synergistic growth inhibition of gemcitabine/cisplatin and gemcitabine/etoposide on a human lung cancer cell line, RERF-LCOK. Proc AACR 36:a2425, 1995 (abstract)
Jensen PB, Holm B, Sorensen M, Christensen IJ, Sehested M: In vitro cross-resistance and collateral sensitivity in seven resistant small cell lung cancer cell lines: preclinical identification of suitable drug partner to taxotere, taxol, topotecan and gemcitabine. Brit J Cancer 75:869–877, 1997
Van Moorsel CJA, Pinedo HM, Veerman G, Guechev A, Smid K, Loves WJ, Vermorken JB, Postmus PE, Peters GJ: Combination chemotherapy studies with gemcitabine and etoposide in non-small cell lung and ovarian cancer cell lines. Biochem Pharmacol 57:407–415, 1999
Mok TS, Zee B, Chan AT, Yeo W, Yang WT, Yim A, Leung SF, Nguyen B, Leung TW, Johnson P: A phase II study of gemcitabine plus oral etoposide in the treatment of patients with advanced non-small cell lung carcinoma. Cancer 89:543–550, 2000
Al-Jazayrly G, Gandara D, Tanaka M, Meyers F, Wun T, Drakes T, Edelman M, Richman E, Richman C, Sy M, Lara P, Lim N, Gummaraju S, Christensen S, Houston J, Lauder I, Lau D: Intrapatient dose escalation of gemcitabine and oral etoposide in advanced solid tumors. Proc ASCO 17:a207, 1998 (abstract)
Hoskins PJ, Swenerton KD: Oral etoposide is active against platinum-resistant epithelial ovarian cancer. J Clin Oncol 12:60–63, 1994
Marzola M, Zucchetti M, Colombo N, Sessa C, Pagani O, D'Incalci M, Cavalli F, Mangioni C: Low-dose oral etoposide in epithelial ovarian cancer of the ovary. Ann Oncol 4:517–519, 1993
Joel SP, Shah R, Slevin ML: Etoposide dosage and pharmacodynamics. Cancer Chemother Pharmacol 34:S69–S75, 1994
D'Incalci M, Rossi C, Zucchetti M, Urso R, Cavalli F, Mangioni C, Willems Y, Sessa C: Pharmacokinetics of etoposide in patients with abnormal renal and hepatic function. Cancer Res 46:2566–2571, 1986
Pfluger KH, Hahn M, Holz JB, Schmidt L, Kohl P, Fritsch HW, Jungclas H, Havemann K: Pharmacokinetics of etoposide: correlation of pharmacokinetic parameters with clinical conditions. Cancer Chemother Pharmacol 31: 350–356, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garcia, A.A., Bookman, M.A., Rodriguez-Rodriguez, L. et al. Phase I Escalation of Gemcitabine Combined with Protracted Oral Etoposide in Gynecologic Malignancies – A Gynecologic Oncology Group Study. Invest New Drugs 20, 383–387 (2002). https://doi.org/10.1023/A:1020633300898
Issue Date:
DOI: https://doi.org/10.1023/A:1020633300898