Abstract
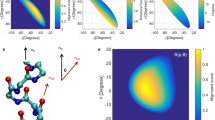
The Cα chemical shift tensors of proteins contain information on the backbone conformation. We have determined the magnitude and orientation of the Cα chemical shift tensors of two peptides with α-helical torsion angles: the Ala residue in G*AL (φ=−65.7°, ψ=−40°), and the Val residue in GG*V (φ=−81.5°, ψ=−50.7°). The magnitude of the tensors was determined from quasi-static powder patterns recoupled under magic-angle spinning, while the orientation of the tensors was extracted from Cα–Hα and Cα–N dipolar modulated powder patterns. The helical Ala Cα chemical shift tensor has a span of 36 ppm and an asymmetry parameter of 0.89. Its σ11 axis is 116° ± 5° from the Cα–Hα bond while the σ22 axis is 40° ± 5° from the Cα–N bond. The Val tensor has an anisotropic span of 25 ppm and an asymmetry parameter of 0.33, both much smaller than the values for β-sheet Val found recently (Yao and Hong, 2002). The Val σ33 axis is tilted by 115° ± 5° from the Cα–Hα bond and 98° ± 5° from the Cα–N bond. These represent the first completely experimentally determined Cα chemical shift tensors of helical peptides. Using an icosahedral representation, we compared the experimental chemical shift tensors with quantum chemical calculations and found overall good agreement. These solid-state chemical shift tensors confirm the observation from cross-correlated relaxation experiments that the projection of the Cα chemical shift tensor onto the Cα–Hα bond is much smaller in α-helices than in β-sheets.
Similar content being viewed by others
References
Alderman, D.W., Sherwood, M.H. and Grant, D.M. (1993) J. Magn. Reson., A101, 188–197.
Bennett, A.E., Rienstra, C.M., Auger, M., Lakshmi, K.V. and Griffin, R.G. (1995) J. Chem. Phys., 103, 6951–6958.
Bower, P.V., Oyler, N., Mehta, M.A., Long, J.R., Stayton, P.S. and Drobny, G.P. (1999) J. Am. Chem. Soc., 121, 8373–8375.
Carroll, P.J., Stewart, P.L. and Opella, S.J. (1990) Acta Cryst., C46, 243–246.
Chaturvedi, S., Go, K. and Parthasarathy, R. (1991) Biopolymers, 31, 397–407.
Costa, P.R., Gross, J.D., Hong, M. and Griffin, R.G. (1997) Chem. Phys. Lett., 280, 95–103.
Dixon, W.T. (1982) J. Chem. Phys., 77, 1800–1809.
Duncan, T.M. (1997) Chemical Shift Tensors, 2nd edn, Farragut Press, Madison, Wisconsin.
Feng, X., Eden, M., Brinkmann, A., Luthman, H., Eriksson, L., Graslund, A., Antzutkin, O.N. and Levitt, M.H. (1997) J. Am. Chem. Soc., 119, 12006–12007.
Harris, R.K. and Oliveri, A.C. (1992) Prog. NMR Spectrosc., 24, 435–456.
Hartzell, C.J., Pratum, T.K. and Drobny, G. (1987) J. Chem. Phys., 87, 4324–4331.
Hartzell, C.J., Whitfeld, M., Oas, T.G. and Drobny, G.P. (1987) J. Am. Chem. Soc., 109, 5966–5969.
Havlin, R.H., Laws, D.D., Bitter, H.L., Sanders, L.K., Sun, H., Grimley, J.S., Wemmer, D.E., Pines, A. and Oldfield, E. (2001) J. Am. Chem. Soc., 123, 10362–10369.
Havlin, R.H., Le, H., Laws, D.D., deDios, A.C. and Oldfield, E. (1997) J. Am. Chem. Soc., 119, 11951–11958.
Hong, M. (2000) J. Am. Chem. Soc., 122, 3762–3770.
Hong, M., Gross, J.D. and Griffin, R.G. (1997) J. Phys. Chem., B101, 5869–5874.
Jameson, A.K. and Jameson, C.J. (1987) Chem. Phys. Lett., 134, 461–466.
Jameson, C.J. (1998) Solid State NMR, 11, 265–268.
Lalitha, V., Subramanian, E. and Bordner, J. (1984) Int. J. Pept. Protein Res., 24, 437–441.
Liu, S.F., Mao, J.D. and Schmidt-Rohr, K. (2002) J. Magn. Reson., 155, 15–28.
Oldfield, E. (1995) J. Biomol. NMR, 5, 217–225.
Schmidt-Rohr, K. and Spiess, H.W. (1994) Multidimensional Solid-State NMR and Polymers, Academic Press, San Diego, California.
Sitkoff, D. and Case, D.A. (1998) Prog. NMR Spectrosc., 32, 165–190.
Spera, S. and Bax, A. (1991) J. Am. Chem. Soc., 113, 5490–5492.
Sun, H., Sanders, L.K. and Oldfield, E. (2002) J. Am. Chem. Soc., submitted.
Tjandra, N. and Bax, A. (1997) J. Am. Chem. Soc., 119, 9576–9577.
Tycko, R., Dabbagh, G. and Mirau, P. (1989) J. Magn. Reson., 85, 265–274.
Walling, A.E., Pargas, R.E. and deDios, A.C. (1997) J. Phys. Chem., A101, 7299–7303.
Weliky, D. and Tycko, R. (1996) J. Am. Chem. Soc., 118, 8487–8488.
Wishart, D.S., Sykes, B.D. and Richards, F.M. (1991) J. Mol. Biol., 222,311–333.
Yao, X.L. and Hong, M. (2002) J. Am. Chem. Soc., 124, 2730–2738.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, X., Yamaguchi, S. & Hong, M. Cα chemical shift tensors in helical peptides by dipolar-modulated chemical shift recoupling NMR. J Biomol NMR 24, 51–62 (2002). https://doi.org/10.1023/A:1020626802472
Issue Date:
DOI: https://doi.org/10.1023/A:1020626802472