Abstract
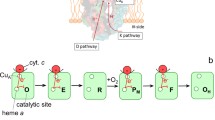
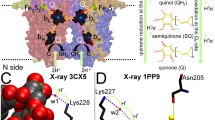
During the last few years our knowledge of the structure and function of heme copper oxidases has greatly profited from the use of site-directed mutagenesis in combination with biophysical techniques. This, together with the recently-determined crystal structures of cytochrome c oxidase, has now made it possible to design experiments aimed at targeting specific pump mechanisms. Here, we summarize results from our recent kinetic studies of electron and proton-transfer reactions in wild-type and mutant forms of cytochrome c oxidase from Rhodobacter sphaeroides. These studies have made it possible to identify amino acid residues involved in proton transfer during specific reaction steps and provide a basis for discussion of mechanisms of electron and proton transfer in terminal oxidases. The results indicate that the pathway through K(I-362)/T(I-359), but not through D(I-132)/E(I-286), is used for proton transfer to a protonatable group interacting electrostatically with heme a 3, i.e., upon reduction of the binuclear center. The pathway through D(I-132)/E(I-286) is used for uptake of pumped and substrate protons during the pumping steps during O2 reduction.
Similar content being viewed by others
REFERENCES
Ädelroth, P., Brzezinski, P., and Malmström, B. G. (1995). Biochemistry 34, 2844-2849.
Ädelroth, P., Sigurdson, H., Hallén, S., and Brzezinski P. (1996). Proc. Natl. Acad. Sci. USA 93, 12292-12297.
Ädelroth, P., Svensson Ek, M., Mitchell, D.M., Gennis, R. B., and Brzezinski, P. (1997). Biochemistry 36, 13824-13829.
Ädelroth, P., Gennis, R. B., and Brzezinski, P. (1998). Biochemistry 37, 2470-2476.
Akeson, M., and Deamer, D. W. (1991). Biophys. J. 60, 101-109.
Babcock, G. T., and Wikström, M. (1992). Nature 356, 301-309.
Babcock, G. T., Floris, R., Nilsson, T., Pressler, M., Varotsis, C., and Vollenbroek, E. (1996). Inorg. Chim. Acta 243, 345-353.
Baciou, L., and Michel, H. (1995). Biochemistry 34, 7967-7972.
Brzezinski, P. (1996). Biochemistry 35, 5611-5615.
Fann, Y. C., Ahmed, I., Blackburn, N. J., Boswell, J. S., Verkhovskaya, M. L., Hoffman, B.M., and Wikström, M. (1995). Biochemistry 34, 10245-10255.
Ferguson-Miller, S., and Babcock, G. T. (1996). Chem. Rev. 96, 2889-2907.
Fetter, J. R., Qian, J., Shapleigh, J., Thomas, J. W., Garcia-Horsman, A., Schmidt, E., Hosler, J., Babcock, G. T., Gennis, R. B., and Ferguson-Miller, S. (1995). Proc. Natl. Acad. Sci. USA 92, 1604-1608.
Fetter, J. R., Sharpe, M., Qian, J., Mills, D., Ferguson-Miller, S., and Nicholls, P. (1996). FEBS Lett. 393, 155-160.
Gibson, Q. H., and Greenwood, C. (1963). Biochem. J. 86, 541-554.
Gutman, M., and Nachliel, E. (1990). Biochim. Biophys. Acta 1015, 391-414.
Hallén, S., and Brzezinski, P. (1994). Biochim. Biophys. Acta 1184, 207-218.
Hallén, S., Brzezinski, P., and Malmström, B. G. (1994). Biochemistry 33, 1467-1472.
Han, S, Ching, Y. C., and Rousseau, D. L. (1990). Nature 348, 89-90.
Hill, B. C. (1993). J. Bioenerg. Biomembr. 25, 115-120.
Hofacker, I., and Schulten, K. (1998). Proteins 30, 100-107.
Hosler, J. P., Shapleigh, J. P., Mitchell, D. M., Kim, Y., Pressler, M. A., Georgiou, C., Babcock, G. T., Alben, J. O., Ferguson-Miller, S., and Gennis, R. B. (1996). Biochemistry 35, 10776-10783.
Iwata, S., Ostermeier, C., Ludwig, B., and Michel, H. (1995). Nature 376, 660-669.
Jünemann, S., Meunier, B., Gennis, R. B., and Rich, P. R. (1997). Biochemistry 36, 14456-14464.
Konstantinov, A. A., Siletsky, S., Mitchell, D., Kaulen, A., and Gennis, R. B. (1997). Proc. Natl. Acad. Sci. USA 94, 9085-9090.
Lanne, B., Malmström, B.G., and Vänngard, T. (1979). Biochim. Biophys. Acta 545, 205-214.
Lanyi, J.K. (1993). Biochim. Biophys. Acta 1183, 241-261.
Martinez, S.E., Huang, D., Ponomarev, M., Cramer, W.A., and Smith, J.L. (1996). Protein Sci. 5, 1081-1092.
Mitchell, D.M., Aasa, R., Ädelroth, P., Brzezinski, P., Gennis, R.B., and Malmström, B.G. (1995). FEBS Lett. 374, 371-374.
Mitchell, R., and Rich, P. R. (1994). Biochim. Biophys. Acta 1186, 19-26.
Nagle, J.F., and Morowitz, H.J. (1978). Proc. Natl. Acad. Sci. USA 75, 298-302.
Oliveberg M., Hallén, S., and Nilsson, T. (1991). Biochemistry 30, 436-440.
Pomès, R., and Roux, B. (1997). Biophys. J. 71, 19-39.
Qian, J., Shi, W., Pressler, M., Hoganson, C., Mills, D., Babcock, G.T., and Ferguson-Miller, S. (1997). Biochemistry 36, 2539-2543.
Regan, J. J., Ramirez, B. E., Winkler, J. R., Gray, H. B., and Malmstrom, B. G. (1998). J. Bioenergetics and Biomembranes 30, 35-40.
Riistama, S., Hummer, G., Puustinen, A., Dyer, B.R., Woodruff, W.H., and Wikström, M. (1997). FEBS Lett. 414, 275-280.
Schulten, Z., and Schulten, K. (1985). Eur. Biophys. J. 11, 149-155.
Svensson M., Hallén, S., Thomas, J. W., Lemieux, L. J., Gennis, R. B., and Nilsson, T. (1995). Biochemistry 34, 5252-5258.
Svensson Ek, M., and Brzezinski, P. (1997). Biochemistry 36, 5425-5431.
Svensson Ek, M., Thomas, J.W, Gennis, R.B, Nilsson, T, and Brzezinski, P. (1996). Biochemistry 35, 13673-13680.
Thomas, J.W., Puustinen, A., Alben, J.O., Gennis, R.B., and Wikström, M. (1993). Biochemistry 32, 10923-10928.
Tsukihara, T., Aoyama, H., Yamashita, E., Tomizaki, T., Yamaguchi, H., Shinzava-Itoh, K., Nakashima, R., Yaono, R., and Yoshikawa, S. (1995). Science 269, 1069-1074.
Tsukihara, T., Aoyama, H., Yamashita, E., Tomizaki, T., Yamaguchi, H., Shinzava-Itoh, K., Nakashima, R., Yaono, R., and Yoshikawa, S. (1996). Science 272, 1136-1144.
Varotsis, C., Zhang, Y., Appelman, E.H., and Babcock, G.T. (1993). Proc. Natl. Acad. Sci. USA 90, 237-241.
Verkhovsky, M. I., Morgan, J. E., Verkhovskaya, M. L., and Wikström, M. (1997). Biochim. Biophys. Acta 1318, 6-10.
Wikström, M. (1988). Chemi. Scr. 28A, 71-74.
Wikström, M. (1989). Nature 338, 776-778.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brzezinski, P., Ädelroth, P. Pathways of Proton Transfer in Cytochrome c Oxidase. J Bioenerg Biomembr 30, 99–107 (1998). https://doi.org/10.1023/A:1020567729941
Issue Date:
DOI: https://doi.org/10.1023/A:1020567729941