Abstract
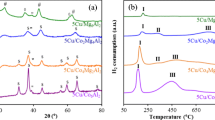
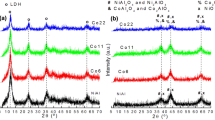
Hydrogen (H2) is expected to become an important fuel for the future to be used as an energy carrier in automobiles and electric power plants. A promising route for H2 production involves catalytic reforming of a suitable primary fuel such as methanol or ethanol. Since ethanol is a renewable raw material and can be cheaply produced by the fermentation of biomass, the ethanol reforming for H2 production is beneficial to the environment. In the present study, the steam reforming of ethanol in the presence of added O2, which in the present study is referred to as oxidative steam reforming of ethanol (OSRE), was performed for the first time over a series of CuNiZnAl mixed oxide catalysts derived from layered double hydroxide (LDH) precursors. The effects of Cu/Ni ratio, temperature, O2/ethanol ratio, contact time, CO co-feed and substitution of Cu/Ni by Co were investigated systematically in order to understand the influence of these parameters on the catalytic performance. An ethanol conversion close to 100% was noticed at 300 °C over all the catalysts. The Cu-rich catalysts favor the dehydrogenation of ethanol to acetaldehyde. The addition of Ni was found to favor the C–C bond rupture, producing CO, CO2 and CH4. Depending upon the reaction condition, a H2 yield between 2.5 and 3.5 moles per mole of ethanol converted was obtained. A CoNi-based catalyst exhibited better catalytic performance with lower selectivity of undesirable byproducts, namely CH3CHO, CH4 and CO.
Similar content being viewed by others
References
C. Song, Am. Chem. Soc. Fuel Chem. Division Preprint 46 (2001) 8.
L.F. Brown, Int. J. Hydrogen Energy 26 (2001) 381.
J. Rostrup-Nielson, Phys. Chem. Chem. Phys. 3 (2001) 283.
W.-H. Cheng, Acc. Chem. Res. 32 (1999) 685.
J.P. Breen and J.R.H. Ross, Catal. Today 51 (1999) 521.
L. Alejo, R. Lago, M.A. Peña and J.L.G. Fierro, Appl. Catal. A: General 162 (1997) 281.
S. Velu, K. Suzuki, M. Okazaki, M.P. Kapoor, T. Osaki and F. Ohashi, J. Catal. 194 (2000).
S. Velu, K. Suzuki, M.P. Kapoor, F. Ohashi and T. Osaki, Appl. Catal. A: General 213 (2001) 47.
S. Velu, K. Suzuki and T. Osaki, Catal. Lett. 69 (2000) 43.
T.L. Reitz, P.L. Lee, K.F. Czaplewski, J.C. Lang, K.E. Popp and H.H. Kung, J. Catal. 199 (2001) 193.
S. Murcia-Mascaros, R.M. Navarro, L. Gomez-Sainero, U. Costantino, M. Nocchetti and J.L.G. Fierro, J. Catal. 198 (2001) 338.
Biofuels, DOE/GO 10099, 736 (1999).
F. Marino, M. Jobbagy, G. Baronetti and M. Loborde, Stud. Surf. Sci. Catal. 130 (2000) 2147.
F. Marino, M. Boveri, G. Baronetti and M. Loborde, Int. J. Hydrogen Energy 26 (2001) 665.
F. Haga, T. Nakajima, H. Miya and S. Mishima, Catal. Lett. 48 (1997) 223.
K. Vasudeva, N. Mitra, P. Umasanker and S.C. Dhingra, Int. J. Hydrogen Energy 21 (1996) 13.
I. Fishtik, A. Alexander, R. Datta and D. Geana, Int. J. Hydrogen Energy 25 (2000) 31.
A. Yee, S.J. Morrison and H. Idriss, J. Catal. 186 (1999) 279.
F. Cavani, F. Trifiro and A. Vaccari, Catal. Today 11 (1991) 173.
A. Alejandre, F. Medina, X. Rodriguez, P. Salagre and J.E. Sueiras, Stud. Surf. Sci. Catal. 130 (2000) 1763.
A. Monzon, E. Romeo, C. Roya, R. Trujillano, F.M. Labajos and V. Rives, Appl. Catal. A: General 185 (1999) 53.
S. Velu, K. Suzuki, S. Hashimoto, N. Satoh and S. Tomura, J. Mater. Chem. 11 (2001) 2049.
S. Velu, K. Suzuki, M.P. Kapoor, S. Tomura, F. Ohashi and T. Osaki, Chem. Mater. 12 (2000) 719.
N. Iwasa and Takezawa, Bull. Chem. Soc. Jpn. 64 (1991) 2619.
Y. Liu and D. Liu, Int. J. Hydrogen Energy 24 (1999) 351.
M. Agnelli and C. Mirodatos, J. Catal. 192 (2000) 204.
A.N. Fatsikostas, D.I. Kondarides and X.E. Verykios, Chem. Commun. (2001), 851.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Velu, S., Satoh, N., Gopinath, C.S. et al. Oxidative Reforming of Bio-Ethanol Over CuNiZnAl Mixed Oxide Catalysts for Hydrogen Production. Catalysis Letters 82, 145–152 (2002). https://doi.org/10.1023/A:1020516830768
Issue Date:
DOI: https://doi.org/10.1023/A:1020516830768