Abstract
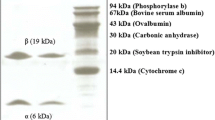
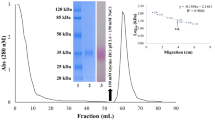
Peanut (Arachis hypogaea) seed lectin, PNA is widely used to identify tumor specific antigen (T-antigen), Galβ1-3GalNAc on the eukaryotic cell surface. The functional amino acid coding region of a cDNA clone, pBSH-PN was PCR amplified and cloned downstream of the polyhedrin promoter in the Autographa californica nucleopolyhedrovirus (AcNPV) based transfer vector pVL1393. Co-transfection of Spodoptera frugiperda cells (Sf9) with the transfer vector, pAcPNA and AcRP6 (a recombinant AcNPV having B-gal downstream of the polyhedrin promoter) DNAs produced a recombinant virus, AcPNA which expresses PNA. Infection of suspension culture of Sf9 cells with plaque purified AcPNA produced as much as 9.8 mg PNA per liter (2.0 × 106 cells/ml) of serum-free medium. Intracellularly expressed protein (re-PNA) was purified to apparent homogeneity by affinity chromatography using ECD-Sepharose. Polyclonal antibodies against natural PNA (n-PNA) cross-reacted with re-PNA. The subunit molecular weight (30kDa), hemagglutination activity, and carbohydrate specificity of re-PNA were found to be identical to that of n-PNA, thus confirming the abundant production of a functionally active protein in the baculovirus expression system.
Similar content being viewed by others
REFERENCES
Lotan, R., Skutelsky, E., Danon, D., and Sharon, N. (1975) J. Biol. Chem. 250:8518-8523.
Pereira, M. E. A., Kabat, E. A., Lotan, R., and Sharon, N. (1976) Carbohydr. Res. 51:107-118.
Salunke, D. M., Swamy, M. J., Khan, M. I., Mande, S. C., Surolia, A., and Vijayan, M. (1985) J. Biol. Chem. 260:13576-13579.
Banerjee, R., et al. (1994) Proc. Natl. Acad. Sci. USA 91:227-231.
De Boek, H., Matta, K. L., Claeyssens, M., Sharon, N., and Loontiens, F. G. (1983) Eur. J. Biochem. 131:453-460.
Swamy, M. J., Gupta, D., Mahanta, S. K., and Surolia, A. (1991) Carbohydr. Res. 213:59-67.
Rittenhouse-Diakun, K., Xia, Z., Pickhardt, D., Morey, S., Baek, M. G., and Roy, R. (1998) Hybridoma 17:165-173.
Sharma, V., Vijayan, M., and Surolia, A. (1996) J. Biol. Chem. 271:21209-21213.
Rodriguez-Arango, E., Arango, R., Adar, R., Galili, G., and Sharon, N. (1992) FEBS Lett. 307:185-189.
Sharma, V. and Surolia, A. (1994) Gene 148:299-304.
Sharon, H. and Lis, H. (1989) “Lectins”, Chapman and Hall, New York.
Sharma, V. and Surolia, A. (1997) J. Mol. Biol. 267:433-445.
Sharon, N. and Lis, H. (1995) Essay Biochem. 30:59-75.
Liener, I. E., Sharon, N., and Goldstein, I. J. (1986) The Lectins: Properties, Functions and Applications in Biology and Medicine. Academic Press, London/Orlando.
Tautz, D. and Renz, M. (1983) Anal. Biochem. 132:14-19.
O'Reily, D. R., Miller, L. K., and Luckow, V. A. (1992) “Baculovirus expression vectors”, Freeman, New York, pp. 38-41.
Summers, M. D. and Smith, G. E. (1987) A Manual of Methods for Baculovirus Vectors and Insect Cell Culture Procedures, Texas Agricultural Station, College Station, Bulletin No. 1555.
Groebe, D. R., Chung, A. E., and Ho, C. (1990) Nucl. Acids. Res. 18:4033.
Das, R. H., Bansal, O. B., Behera, A. K., Durgaprasad, Y., Kumar, M., and Bali, A. (1996) Biotechniques 20:364-368.
Matsura, Y., Possee, R. D. Overton, H. A. and Bishop, D. H. L. (1987) J. Gen. Virol. 68:1233-1250.
Sanger F., Nicklen, S., and Coulson, A. R. (1977) Proc. Natl. Acad. Sci., USA 74:5463-5467.
Rice, J. W. et al. (1993) Biotechniques 15:1052-1059.
Ersson, B., Aspberg, K., and Porath, J. (1973) Biochem. Biophys. Acta 310:446-452.
Laemmli, U. K. (1970) Nature 227:680-685.
Towbin, H., Staehelin, T., and Gordon, J. (1979) Proc. Natl. Acad. Sci. USA 76:4350-4354.
Das, H. R. et al. (1983) Carbohydr. Res. 120:303-314.
Young, N. M., Johnston, A. Z. R., and Watson, D. C. (1991) Eur. J. Biochem. 196:631-637.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kumar, M., Behera, A.K., Kumar, S. et al. Expression, Purification and Characterization of Peanut (Arachis hypogaea) Agglutinin (PNA) from Baculovirus Infected Insect Cells. Biosci Rep 19, 227–234 (1999). https://doi.org/10.1023/A:1020234005099
Issue Date:
DOI: https://doi.org/10.1023/A:1020234005099