Abstract
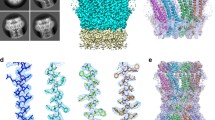
Gap junctions appear to be essential components of metazoan animals providing a means of direct means of communication between neighboring cells. They are sieve-like structures which allow cell–cell movement of cytosolic solutes below 1000 MW. The major role of gap junctions would appear to be homeostatic giving rise to groups of cells which act as functional units. Ductin is the major core component of gap junctions and recent structural data shows it to be a four alpha-helical bundle which fits particularly well into a low resolution model of the gap junction channel. Ductin is also the main membrane component of the vacuolar H+-ATPase that is found in all eukaryotes and it seems likely that the gap junction channel first evolved as a housing for the rotating spindle of these proton pumps. Because ductin protrudes little from the membrane, other proteins are required to bring cell surfaces close enough together to form gap junctions. Such proteins may include connexins, a large family of proteins found in vertebrates.
Similar content being viewed by others
REFERENCES
Revel, J.-P. and Karnovsky, M. J. (1967) Hexagonal arrays of subunits in intercellular junctions of the mouse heart and liver. J. Cell Biol. 33:C7–C12
Weidmann, S. (1952) The electrical constants of Purkinje fibres. J. Physiol. 118:348–360
Furshpan, E. J. and Potter, D. D. (1959) Transmission at the giant motor synapses of the crayfish. J. Physiol. 145:289–235
Loewenstein, W. R. and Kanno, Y. (1964) Studies on the epithelial (gland) cell junction. I modification of surface membrane permeability. J. Cell Biol. 22:565–586
Benenedetti, E. L. and Emmelot, P. (1968) Hexagonal array of subunits in tight junctions separated from isolated rat liver plasma membranes. J. Cell Biol. 38:15–24
Caspar, D. L. D., Goodenough, D. A., Makowski, L., and Phillips, W. C. (1977) Gap junction structures: I correlated electron microscopy and X-ray diffraction. J. Cell Biol. 74:605–628.
Makowski, L., Caspar, D. L. D., Phillips, W. C., and Goodenough, D. A. (1977) Gap junction structures: II analysis of X-ray diffraction data. J. Cell Biol. 74:629–645.
Unwin, P. N. T. and Ennis, P. D. (1984) Two configurations of a channel-forming membrane protein. Nature 307:609–613.
Hoh, J. H., Lal, R., John, S. A., Revel, J.-P., and Arnsdorf, M. F. (1991). Atomic force microscopy and dissection of gap junctions. Science 253:1405–1408.
Leitch, B. and M. E. Finbow (1990) The gap junction-like form of a vacuolar proton channel appears not to be an artefact of isolation: an immunocytochemical localization study. Exp Cell Res. 190:218–226.
Sikerwar, S. S., Downing, K. H., and Glaeser, R. M. (1991). Three-dimensional structure of an invertebrate intercellular communicating junction. J. Structur. Biol. 106:255–263.
Finbow, M. E. et al., (1992) Structure of a 16kD protein that has identity to the putative proton channel of the vacuolar H+-ATPase. Protein Eng 5:7–15.
Holzenburg, A., et al. (1993) Evidence for a common structure for a class of membrane channels, Euro J. Biochem. 213:21–30.
John, S. A., Saner, D., Pitts, J. D., Holzenburg, A., Finbow, M. E., and Lal, R. (1997) Atomic Force Microscopy of Arthropod Gap Junctions. J. Struct. Biol. 120:22–31.
Pitts, J. D. and Simms, J. W. (1977) The permeability of junctions between animal cells. Exp Cell Res. 104:153–163.
Finbow, M. E. and Pitts, J. D. (1980) Permeability of junctions between animal cells. Exp Cell Res. 13:1–13.
Goodenough, D. A., Goliger, J. A., and Paul, D. L. (1996) Connexins, Connexons, and Intercellular Communication Ann. Rev. Biochem. 65:475–502.
Finbow, M. E. and Meagher, L. (1992) Connexins and the vacuolar proteolipid-like 16-kDa protein are not directly associated with each other but may be components of similar or the same gap junctional complex. Exp Cell Res. 203:280–284.
Finbow, M. E. (1997) Vertebrate and Invertebrate Gap Junctions: A common molecular basis? Cell Biol. Int. 21:329–331.
Finbow, M. E. and Pitts, J. D. (1993) Is the gap junctional channel—the connexon—made of connexin or ductin? J. Cell Sci. 106:463–472.
Mandel, M., Moriyama, Y., Hulmes, J. D., Pan, Y.-C., Nelson, H., and Nelson, N. (1988). cDNA sequence encoding the 16-kDa proteolipid of chromaffin granules implies gene duplication in the evolution of H+-ATPases. Proc. Natl. Acad. Sci. USA 85:5521–5524.
Finbow, M. E. and Harrison, M. A. (1997) The vacuolar H+-ATPase-a universal proton pump of eukaryotes. Biochem. J. 324:997–1712.
Boyer, P. D. (1997) The ATP synthase—a splendid molecular machine. Ann. Rev. Biochem. 66:717–749.
Stevens, T. H. and Forgac, M. (1997) Structure, function and regulation of the vacuolar (H+)-ATPase. Ann. Rev. Cell Dev. Biol. 13:779–808.
Girvin, M. E., Rastogi, V. K., Abidgaard, F., Markley, J. L., and Fillinghame, R. H. (1998) Solution structure of the transmembrane H+-transporting subunit c of the F 1 F 0 ATP synthase. Biochemistry 37:8817–8824.
Rahlfs, S. and Muller, V. (1997) Sequence of subunit c of the Na(+)-translocating F 1 F 0 ATPase of Acetobacterium woodii: proposal for determinants of Na+ specificity as revealed by sequence comparisons. FEBS Letts 404:269–271.
Hilario, E. and Gogarten, J. P. (1998) The Prokaryote-to-Eukaryote Transition Reflected in the Evolution of the V/F/A-ATPase Catalytic and Proteolipid Subunits. J. Mol. Evol. 46:703–715.
Finbow, M. E., John, S., Kam, E., Apps, D. K., and Pitts, J. D. (1993) Disposition and orientation of Ductin (DCCD reactive vacuolar H+-ATPase subunit) in mammalian membrane complexes. Exp Cell Res. 207:261–270.
Jones, P. C., Harrison, M. A., Kim, Y.-I., Finbow, M. E., and Findlay, J. B. C. (1995) The first putative transmembrane helix of the 16 kDa proteolipid lines a pore in the V 0 sector of the vacuolar H+-ATPase. Biochem. J., 312:9211–9218.
Hughes, G., Harrison, M. A., Kim, Y.-I., Griffiths, D. E., Finbow, M. E., and Findlay, J. B. C. (1996) Interaction of dibutylin-3-phydroxyflavone bromide with the 16 kDa proteolipid indicates the disposition of proton translocation sites of the vacuolar ATPase. Biochem. J., 317:425–431.
Dunlop, J., Jones, P. C., and Finbow, M. E. (1995) Membrane insertion and assembly of ductin: a polytopic channel with dual orientations. EMBO J. 14:3609–3616.
Phelan, P., Stebbings, L. A., Baines, R. A., Bacon, J. P., Davies, J. A., and Ford, C. (1998) Drosophila Shaking B protein forms gap junctions in paired Xenopus oocytes. Nature 391:181–184.
Hertzberg, E. L., Spray, D. C., and Bennett, M. V. L. (1985) Reduction of gap junctional conductance by micro-injection of antibodies to the 27-kDa liver gap junction polypeptide. Proc. Natl. Acad. Sci. USA 82:2412–2416.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Finbow, M.E., Pitts, J.D. Structure of the Ductin Channel. Biosci Rep 18, 287–297 (1998). https://doi.org/10.1023/A:1020205231507
Issue Date:
DOI: https://doi.org/10.1023/A:1020205231507