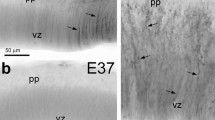
Abstract
The mechanisms that control the production and differentiation of glial cells during development are difficult to unravel because of displacement of precursor cells from their sites of origin to their permanent location. The two main neuroglial cells in the rat spinal cord are oligodendrocytes and astrocytes. Considerable evidence supports the view that oligodendrocytes in the spinal cord are derived from a region of the ventral ventricular zone (VZ). Some astrocytes, at least, may arise from radial glia. In this study a 5-Bromo-2′-deoxyuridine (BrdU) incorporation assay was used to identify proliferating cells and examine the location of proliferating glial precursor cells in the embryonic spinal cord at different times post BrdU incorporation. In this way the migration of proliferating cells into spinal cord white matter could be followed. At E14, most of the proliferating cells in the periventricular region were located dorsally and these cells were probably proliferating neuronal precursors. At E16 and E18, the majority of the proliferating cells in the periventricular region were located ventrally. In the white matter the number of proliferating cells increased as the animals increased in age and much of this proliferation occurred locally. BrdU labelling showed that glial precursor cells migrate from their ventral and dorsal VZ birth sites to peripheral regions of the cord. Furthermore although the majority of proliferating cells in the spinal cord at E16 and E18 were located in the ventral periventricular region, some proliferating cells remained in the dorsal VZ region of the cord.
Similar content being viewed by others
References
Altman, J. & Bayer, S. A. (1984) The development of the rat spinal cord. In Advances in Anatomy, Embryology and Cell Biology, Vol. 85 (edited by Beck, J. F.). Berlin: Springer-Verlag.
Calver, A. R., Hall, A. L., Yu, W.-P., Walsh, F. S., Heath, J. K., Betsholtz, C. & Richardson, W. D. (1998) Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron 20, 869–882.
Cameron-Curry, P. & le Douarin, N. M. (1995) Oligodendrocyte precursors originate from both the dorsal and the ventral parts of the spinal cord. Neuron 15, 1299–1310.
Das, G. D. (1979) Gliogenesis and ependymogenesis during embryonic development of the rat. Journal of the Neurological Sciences 43, 193–204.
Frederikson, K. & McKay, R. D. G. (1988) Proliferation and differentiation of rat neuroepithelial precursor cell in vivo. Journal of Neuroscience 8, 1144–1151.
Fujita, S. (1965) An autoradiographic study on the origin and fate of the sub-pial glioblast in the embryonic chick spinal cord. Journal of Comparative Neurology 124, 51–60.
Gao, F. B. & Raff, M. (1997) Cell size control and a cell-intrinsic maturation program in proliferating oligodendrocyte precursor cells. Journal of Cell Biology 138, 1367–1377.
Gilmore, S. A. (1971) Neuroglial population in the spinal white matter of neonatal and early postnatal rats: An autoradiographic study of numbers of neuroglia and changes in their proliferative activity. Anatomical Records 171, 283–292.
Gressens, P., Richelme, C., Kadihm, S. S., Gadisseux, J.-F. & Evrard, P. (1992) The germinative zone produces the most cortical astrocytes after neuronal migration in the developing mammalian brain. Biology of Neonate 61, 4–24.
Hall, A., Giese, N. A. & Richardson, W. D. (1996) Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that expressPDGF alpha-receptors. Development 122, 4085–4094.
Hardy, R. J. & Friedrich, V. Jr. (1996) Oligodendrocyte progenitors are generated throughout the embryonic mouse brain, but differentiate in restricted foci. Development 122, 2059–2069.
Jaworski, D. M., Kelly, G. M. & Hockfield, S. (1995) The CNS-specific hyaluronan-binding protein BEHAB is expressed in ventricular zones coincident with gliogenesis. Journal of Neuroscience 15, 1352–1362.
Kessaris, N., Pringle, N. & Richardson, W. D. (2001) Ventral neurogenesis and the neuron-glial switch. Neuron 31, 677–680.
Leber, S. M., Breedlove, S. M. & Sanes, J. R. (1990) Lineage, arrangement and death of clonally related motor neurons in chick spinal cord. Journal of Neuroscience 10, 2451–2462.
Leber, S. M. & Sanes, J. R. (1995) Migratory paths of neurons and glia in the embryonic chick spinal cord. Journal of Neuroscience 15, 1236–1248.
Levison, S. W., Chuang, C., Abramson, B. J. & Goldman, J. E. (1993) The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development 119, 611–622.
Levison, S. W. & Goldman, J. E. (1993) Both oligodendrocytes and astrocytes recognised from progenitors in the subventricular zone of postnatal rat forebrain. Neuron 10, 201–212.
Luskin, M. B. & McDermott, K. W. (1994) Divergent lineages for oligodendrocytes and astrocytes originating in the neonatal forebrain subventricular zone. Glia 11, 211–226.
Mayhew, T. M. (1989) Stereological studies of rat spinal neurons during postnatal development: Estimates of mean perikaryal and nuclear volumes free from assumptions about shape. Journal of Anatomy 162, 97–109.
Mayhew, T. M. (1992) Areview of recent advances in stereology for quantifying neural structure. Journal of Neurocytology 21, 313–328.
McConnell, S. K. (1995) Strategies for the generation of neuronal diversity in the developing central nervous system. Journal of Neuroscience 15, 6987–6998.
McDermott, K. W. & Lantos, P. L. (1990) Cell proliferation in the subependymal layer of the postnatal marmoset, Callithrix jacchus. Developmental Brain Research 57, 269–277.
Miller, M. W. & Nowakowski, R. S. (1988) Use of bromodeoxyuridine immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Research 457, 44–52.
Misson, J.-P., Austin, C. P., Takahashi, T., Cepko, C. L. & Caviness, V. S. Jr. (1991) The alignment of migrating neural cells in relation to the murine neopallial radial glial fibre system. Cerebral Cortex 1, 221–229.
Mizuguchi, R., Sugimori, M., Takebayashi, H., Kosako, H., Nagao, M., Yoshida, S., Nabeshima, K. & Nakafuku, M. (2001) Conbinatorial roles of olig2 and neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motorneurons. Neuron 31, 757–771.
Noll, E. & Miller, R. H. (1993) Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline region in the embryonic rat spinal cord. Development 118, 563–573.
Noll, E. & Miller, R. H. (1994) Regulation of oligodendrocyte differentiation: A role for retinoic acid in the spinal cord. Development 120, 649–660.
Nornes, H. O. & Das, G. P. (1974) Temporal pattern of neurogenesis in spinal cord of rat. 1. Autoradiographic study-time and sites of origin and migration and settling patterns of neuroblasts. Brain Research 73, 121–138.
Novitch, B. G., Chen, A. I. & Jessell, T. M. (2001) Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron 31, 773–789.
O'Brien, D., Dockery, P., McDermott, K. & Fraher, J. P. (1998) The ventral motorneurone axon bundle in the CNS-a cordone system? Journal of Neurocytology 27, 247–258.
Ono, K., Bansal, R., Payne, J., Rutishauser, U. & Miller, R. H. (1995) Early development and dispersal of oligodendrocyte precursors in the embryonic chick spinal cord. Development 121, 1743–1754.
O'Rourke, N. A., Dailey, M. E., Smith, S. J. & McConnell, S. K. (1992) Diverse migratory pathways in the developing cerebral cortex. Science 258, 299–302.
Oudega, M., Lakke, E., Marani, E. & Thomeer, R. (1993) A survey of the development of the rat spinal cord. In Development of the Rat Spinal Cord: Immuno-and Enzyme Histochemical Approaches (edited by Beck, F., Wild, W., Pauly, J. E., Sano, Y. & Schiebler, T. H.). Berlin: Springer-Verlag.
Pringle, N. P., Guthrie, S., Lumsden, A. & Richardson, W. D. (1998) Dorsal Spinal cord neuroepithelium generates astrocytes but not oligodendrocytes. Neuron 20, 883–893.
Pringle, N. P. & Richardson, W. D. (1993) A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development 117, 525–533.
Pringle, N. P., Yu, W. P., Guthrie, S., Roelink, H., Lumsden, A., Peterson, A. C. & Richardson, W. D. (1996) Determination of neuroepithelial cell fate: Induction of the oligodendroglial lineage by ventral midline cells and sonic hedgehog. Developmental Biology 177, 30–42.
Rao, M. S. & Mayer-Proschel, M. (1997) Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Developmental Biology 188, 48–63.
Rao, M. S., Noble, M. & Mayer-Proschel, M. (1998) Atripotential glial precursor is present in the developing spinal cord. Proceedings of the National Academy of Sciences of the USA 95, 3996–4001.
Reznikov, K., Acklin, S. E. & van der Kooy, D. (1997) Clonal heterogeneity in the early embryonic rodent cortical germinal zone and the separation of subventricular fromventricular zone lineages. Developmental Dynamics 210, 328–343.
Richardson, W. D., Smith, H. K., Sun, T., Pringle, N. P., Hall, A. & Woodruff, R. (2000) Oligodendrocyte lineage and the motor neuron connection. Glia 29, 136–142.
Sims, T. J. & Vaughn, J. E. (1979) The generation of neurons involved in an early reflex pathway of embryonic mouse spinal cord. Journal of Comparative Neurology 183, 707–719.
Soula, C., Danesin, C., Kan, P., Grob, M., Poncet, C., & Cochard, P. (2001) Distinct sites of origin of oligodendrocytes and somatic motor neurons in the chick spinal cord: Oligodendrocytes arise from Nkx2.2-expressing progenitors by a Shh-dependent mechanism. Development 128, 1369–1379.
Sturrock, R. R. (1982) Gliognesis in the prenatal rabbit spinal cord. Journal of Anatomy 134, 771–793.
Timsit, S., Martinez, S., Allinquant, B., Peyron, F., Puelles, L. & Zalc, B. (1995) Oligodendrocytes originate in a restricted zone of the embryonic neural tube defined by DM-20 mRNA expression. Journal of Neuroscience 15, 1012–1024.
Warf, B. C., Fok-Seang, J. & Miller, R. H. (1991) Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord. Journal of Neuroscience 11, 2477–2488.
Weidenheim, K. M., Epshteyn, I., Rashbaum, W. K. & Lyman, W. D. (1994) Patterns of glial development in the human foetal spinal cord during the late first and second trimester. Journal of Neurocytology 23, 343–353.
Williams, M. A. (1977) Quantitative methods in biology. In Practical Methods in Electron Microscope Analysis, Vol. 6 (edited by Glauert, A. M.). Amsterdam: Elsevier/North Holland.
Yu, W.-P., Collirini, E. J., Pringle, N. P. & Richardson, W. D. (1994) Embryonic expression of myelin genes: Evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron 12, 1353–1362.
Zhou, Q., Choi, G. & Anderson, D. J. (2001) The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron 31, 791–807.
Rights and permissions
About this article
Cite this article
McMahon, S., McDermott, K. Proliferation and migration of glial precursor cells in the developing rat spinal cord. J Neurocytol 30, 821–828 (2001). https://doi.org/10.1023/A:1019693421778
Issue Date:
DOI: https://doi.org/10.1023/A:1019693421778