Abstract
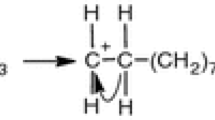
The transformation of ethanol into ether and ethylene was studied over a series of aluminophosphates and silicoaluminophosphates with AFI and AEL topology, at 593 K. It was found that the data followed a simple parallel kinetic scheme. The formation of ether, the less demanding reaction, can be strongly limited by thermodynamics. Based on both the kinetic model and the equilibrium curve for the system considered, a series of parameters were defined in order to determine the relative strength and concentration of the active centres participating in both reactions. Differences in the average specific-activity for the ethanol transformation into ethylene (turnover-like number) were rationalised in terms of differences in the average hydrogen-atoms partial-charge. Structural influence on product distribution due to the shape-selective phenomenon was not observed under the reaction conditions employed.
Similar content being viewed by others
References
C. de las Pozas, R. López-Cordero, J. Gonzalez-Morales, N. Travieso and R. Roque-Malherbe, J. Mol. Catal. 83 (1993) 145.
M. Makarova, E. Paukshits, J.M. Thomas, C. Williams and K.I. Zamaraev, J.Catal. 149 (1994) 36.
E. Derouane, J. Nagy, P. Dejaifve, J. van Hooff, B. Spekman, J.Védrine and C.Naccache, J.Catal. 53 (1978) 40.
E. Costa, A. Uguina, J. Aguado and P. Hernández, Ind. Eng. Chem. Proc.Des. Dev. 24 (1985) 239.
D. Marcano and L. Cortés, in: Química Orgaínica, Vol. 1, ed. Reverté (1982) p. 494.
D. Jingfa, Z. Guirong, D. Shuzhong, P. Haishui and W.Huaiming,Appl. Catal. 41 (1988) 13.
A. Cobo and M. Mendes, Proc. X Iber. Simp. Catal., Vol II (1986) p. 627.
S. Bun, S. Nishiyama, S. Tsuruya and M. Masai, Appl. Catal. 59 (1990) 13.
H.Knözinger and R.Köhne, J.Catal. 5 (1966) 264.
M. Alfonzo, J. Goldwasser, C.M. López, F.J. Machado, M. Matjushin, B. Méndez and M.M. Ramírez de Agudelo, J. Mol.Catal. 98 (1995) 35.
C.A. Emeis, J.Catal. 141 (1993) 347.
D. Stull, E. Westrun and G. Sinke: The Chemical Thermodynamics of Organic Compounds (Wiley, New York, 1969).
J.Martens, P.Grobet and P. Jacobs, J. Catal. 126 (1990) 299.
Chr. Minchev, Ya. Neinska, V. Valtchev, V. Minkov, T. Tsoncheva, V. Penchev, H. Lechert and M. Hess, Catal. Lett. 18 (1993) 125
S. Elangovan, B. Arabindoo, V. Krishnasamy and V.Murugesan, J. Chem. Soc. Faraday Trans. 91 (1995) 4471.
N. Tapp, N. Milestone, M. Bowden and R. Meinhold, Zeolites 10 (1990) 105.
M.L. Cubeiro, C.M. López, A. Colmenares, L. Teixeira, M. Goldwasser, M.J. Perez-Zurita, F.J. Machado and F.González-Jiménez, Appl. Catal., submitted. [18] R. Borade and A. Clearfield, J.Mol. Catal. 88 (1994) 249. [19] V. Choudhary and D. Akolekar, J.Catal. 103 (1987) 115. [20] M. Burgers and H. van Bekkum, Stud. Surf. Sci. Catal. 78 (1993) 567, and references 6, 8 and 9 therein. [21] J. Horsley, E. Derouane, H. Foley, P. Jacobs and R. Szostak, in: Nonaluminosilicate Molecular Sieves, Study Number 4192 MS, Catalytica StudiesDivision (1992) p. 58.
R. Borade andA. Clearfield, J.Mol. Catal. 88 (1994) 249.
V. Choudhary andD. Akolekar, J.Catal. 103 (1987) 115.
M. Burgers and H. van Bekkum, Stud. Surf. Sci. Catal. 78 (1993)567, and references 6, 8 and 9 therein.
J. Horsley, E. Derouane, H. Foley, P. Jacobs and R. Szostak, in: Nonaluminosilicate Molecular Sieves, Study Number 4192 MS,Catalytica StudiesDivision (1992) p. 58.
W.J.Mortier, J. Catal. 55 (1978) 138.
L. Yang, Y. Aishen and X.Qinhua,Appl. Catal. 67 (1991) 169.
J. Campelo, F. Lafoni and J. Marinas, in: Proc. XIII Iber. Simp. Catal.,Vol. II (1992) p. 1031.
Rights and permissions
About this article
Cite this article
Arias, D., Colmenares, A., Cubeiro, M. et al. The transformation of ethanol over AlPO4 and SAPO molecular sieves with AEL and AFI topology. Kinetic and thermodynamic approach. Catalysis Letters 45, 51–58 (1997). https://doi.org/10.1023/A:1019055309967
Issue Date:
DOI: https://doi.org/10.1023/A:1019055309967