Abstract
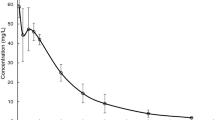
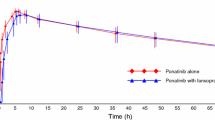
Didanosine is a purine nucleoside analogue approved for the treatment of human immunodeficiency virus infection. It is extremely unstable at pH values less than 3 and requires protection against gastric acid-induced hydrolysis. Beagle dogs pretreated with pentagastrin, an analogue of gastrin that reproducibly stimulates gastric acid secretion, have been used to screen different didanosine formulations. The absolute bioavailability of didanosine from a saline solution decreased from approximately 43% in untreated dogs to 8% after pretreatment with pentagastrin. Administration of buffered solution of didanosine to untreated and pretreated dogs yielded bio-availability estimates of 37 and 30%, respectively. In humans, the bioavailability from a similar buffered solution was approximately 40%. Pentagastrin-pretreated dogs were used to evaluate four new products relative to a citrate-phosphate buffer sachet, the formulation selected for large-scale clinical trials in humans. Two of these new formulations, a chewable tablet and an antacid suspension, were more bioavailable then the reference sachet. This also proved to be true in man, necessitating an adjustment in the dose of didanosine when administered as the chewable tablet. Dogs pretreated with pentagastrin accurately predicted the improved bioavailability of new didanosine formulations prior to clinical use. This animal model may be helpful in evaluating the biopharmaceutics of other acid-labile drugs.
Similar content being viewed by others
REFERENCES
B. D. Anderson, M. B. Wygant, T. X. Xiang, W. A. Waugh, and V. J. Stella. Preformulation solubility and kinetic studies of 2′,3′-dideoxypurine nucleosides: Potential anti-AIDS agents. Int. J. Pharm. 45:27–37 (1988).
C. A. Knupp, W. C. Shyu, R. Dolin, F. T. Valentine, C. McLaren, R. R. Martin, K. A. Pittman, and R. H. Barbhaiya. Pharmacokinetics of didanosine in patients with acquired immunodeficiency syndrome or acquired immunodeficiency syndrome-related complex. Clin. Pharmacol. Ther. 49:523–535 (1991).
S. Kaul, C. A. Knupp, K. A. Dandekar, K. A. Pittman, and R. H. Barbhaiya. Pharmacokinetics of 2′,3′-dideoxyinosine (BMY-40900), a new anti-human immunodeficiency virus agent, after administration of single intravenous doses to beagle dogs. Antimicrob. Agents Chemother. 35:610–614 (1991).
M. Stoltz, M. El-hawari, L. Litle, and P. Alm. Pharmacokinetics and bioavailability of the anti-AIDS drug, 2′,3′-dideoxyinosine (NSC-612049) in beagle dogs. Proc. Am. Assoc. Cancer Res. 29:505 (1988).
M. L. Stoltz, M. El-hawari, L. Litle, A. C. Smith, J. E. Tomaszewski, and C. K. Grieshaber. Pharmacokinetics and bioavailability of 2′,3′-dideoxynucleoside anti-AIDS agents in beagle dogs. Proc. Am. Assoc. Cancer Res. 30:535 (1989).
R. P. Happe and J. J. DeBruijne. Pentagastrin stimulated gastric secretion in the dog (orogastric aspiration technique). Res. Vet. Sci. 33:232–239 (1982).
G. K. McEvoy. Pentagastrin. In American Hospital Formulary Service Drug Information, American Society of Hospital Pharmacists, Inc., MD, 1992, pp. 1399–1400.
C. A. Knupp, F. A. Stancato, E. A. Papp, and R. H. Barbhaiya. Quantitation of didanosine in human plasma and urine by high-performance liquid chromatography. J. Chromatogr. 533:282–290 (1990).
P. Prescott. An approximate test for outliers in linear models. Technometrics 17:129–132 (1975).
S. Riegelman and P. Collier. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J. Pharmacokinet. Biopharm. 8:509–534 (1980).
M. Gibaldi and D. Perrier. Pharmacokinetics, 2nd ed., Marcel Dekker, New York, 1982.
K. C. Kwan and A. E. Till. Novel method for bioavailability assessment. J. Pharm. Sci. 62:1494–1497 (1973).
D. J. Schuirmann. A comparison of the two one-sided test procedure and the power approach for assessing the equivalence of average bioavailability. J. Pharmacok. Biopharm. 15:657–680 (1987).
SAS Institute. SAS User's Guide: Statistics Version, 5th ed., SAS Institute, Inc., Cary, NC, 1985.
J. B. Dressman. Comparison of canine and human gastrointestinal physiology. J. Pharm. Sci. 3:123–131 (1986).
C. Y. Lui, G. L. Amidon, R. R. Berardi, D. Fleisher, C. Youngberg, and J. B. Dressman. Comparison of gastrointestinal pH in dogs and humans: Implications on the use of the beagle dog as a model for oral absorption in humans. J. Pharm. Sci. 75:271–274 (1986).
A. M. Merritt and J. H. Reed. Gastrointestinal function testing. In N. V. Anderson (ed.), Veterinary Gastroenterology, Lea & Febiger, Philadelphia, 1980, pp. 247–262.
L. R. Johnson. Gastric secretion. In L. R. Johnson (ed.), Gastrointestinal Physiology, 2nd ed., Mosby Co., St. Louis, MO, 1981, pp. 55–72.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Knupp, C.A., Shyu, W.C., Morgenthien, E.A. et al. Biopharmaceutics of Didanosine in Humans and in a Model for Acid-Labile Drugs, the Pentagastrin-Pretreated Dog. Pharm Res 10, 1157–1164 (1993). https://doi.org/10.1023/A:1018964117665
Issue Date:
DOI: https://doi.org/10.1023/A:1018964117665