Abstract
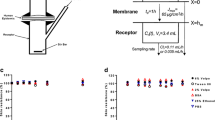
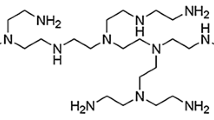
In in vitro skin permeation experiments, the pH of viable epidermis is readily conditioned by the receiver fluid. For weakly ionizable compounds, the flux determined experimentally thus depends on the receiver fluid pH. The purpose of the present work is to characterize this pH effect, since nonphysiological conditions have often been used in the receiver fluid to enhance the solubility of the subject compounds. A transport model was developed to analyze the above-mentioned pH effect of the receiver fluid on the steady state flux of weakly ionizable drugs. The results showed that the skin flux had a strong dependence on pH for those compounds with high intrinsic partition coefficients. Experimentally, this pH effect was observed with a model acid and a model base. The skin flux was found to have a profound dependence on the receiver fluid pH. This dependence also correlates with the octanol/water partition coefficient of the molecule. It was concluded that the use of a physiological receiver fluid would be crucial for a realistic estimation of transdermal potential. The results also suggested that, for weakly ionizable compounds with high partition coefficients, the viable epidermis could be a significant transport barrier for systemic absorption.
Similar content being viewed by others
REFERENCES
I. Diez, H. Colom, J. Moreno, R. Obach, C. Peraire, and J. Domenech. A comparative in vitro study of transdermal absorption of a series of calcium channel antagonists. J. Pharm. Sci. 80:931–934 (1991).
P. P. Sarpotdar, J. L. Gaskill, and R. P. Giannini. Effect of polyethylene glycol 400 on the penetration of drugs through human cadaver skin in vitro. J. Pharm. Sci. 75:26–28 (1986).
A. S. Huq, N. F. H. Ho, N. Husari, G. L. Flynn, W. E. Jetzer, and L. Condie, Jr. Permeation of water contaminative phenols through hairless mouse skin. Arch. Environ. Contam. Toxicol. 15:557–566 (1986).
A. Suzuki, W. I. Higuchi, and N. F. H. Ho. Theoretical model studies of drug absorption and transport in the gastrointestinal tract I. J. Pharm. Sci. 59:644–651 (1970).
D. C. Patel, J. L. Fox, and W. I. Higuchi. Physical model approach in the study of the transport of alkyl amines across a silicone rubber membrane in a two-chamber diffusion cell. J. Pharm. Sci. 73:1028–1033 (1984).
A. Albert and E. P. Serjeant. The Determination of Ionization Constants, a Laboratory Manual, 3rd ed., Chapman and Hall, New York, 1984.
G. L. Flynn, H. Durrheim, and W. I. Higuchi. Permeation of hairless mouse skin. II. Membrane sectioning techniques and influence on alkanol permeabilities. J. Pharm. Sci. 70:52–56 (1981).
W. Hayduk and H. Laudie. Prediction of diffusion coefficients of non-electrolytes in dilute aqueous solutions. A.I.Ch.E. J. 20:611–618 (1974).
S. D. Roy and L. Manoukian. Unpublished result.
C. L. Gu and R. G. Strickley. Preformulation salt selection—Physical property comparisons of the tris(hydroxymethyl)aminomethane (THAM) salts of four analgesic/antiinflammatory agents with the sodium salts and free acids. Pharm. Res. 4:255–257 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kou, J.H., Roy, S.D., Du, J. et al. Effect of Receiver Fluid pH on in Vitro Skin Flux of Weakly lonizable Drugs. Pharm Res 10, 986–990 (1993). https://doi.org/10.1023/A:1018954420874
Issue Date:
DOI: https://doi.org/10.1023/A:1018954420874