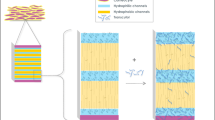
Abstract
Purpose
We investigated the potential correlations between skin barrier integrity and hydrophilic drugs distribution in skin in the presence of different types of penetration enhancers (PEs) and their combinations.
Methods
We measured skin conductivity to evaluate skin barrier integrity before and after the topical application of different chemical PEs, physical PE, peptide PE and their combinations in vitro. We also investigated their effect on the skin distribution profiles of two hydrophilic model drugs, Fluorescein sodium (376 Da) and Fluorescein isothiocyanate-dextrans 10 (10 KDa).
Results
The physical PE significantly increased the skin conductivity compared to all other PEs, while the peptide PE had no effect on it. The drug deposition in different skin layers was not only dependent on PE applied but also its own molecular weight. We further found two excellent correlations: one (R2 = 0.9388) between skin barrier integrity and total skin absorption of FNa and another one(R2 = 0.9212) between skin barrier integrity and the deposition of FNa in dermis and receptor in presence of chemical or physical PEs and their combinations.
Conclusions
The total skin absorption or the deposition in dermis and receptor of small hydrophilic drug in the presence of chemical and physical PEs and their combinations show a good correlation with skin barrier integrity. However, such correlations hold true neither for large hydrophilic drug nor for peptide PE. All good relationships found in this work will allow screening suitable PEs or combinations by measuring the skin conductivity induced by corresponding PEs.

The total skin absorption of small hydrophilic drug shows a good correlation with skin barrier integrity in the presence of chemical and physical penetration enhancers and their combinations. However, such a correlation hold true neither for large hydrophilic drug nor for peptide penetration enhancer.






Similar content being viewed by others
References
Alexander A, Dwivedi S, Ajazuddin TKG, Saraf S, Saraf S, Tripathi DK. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release. 2012;164:26–40.
Schoellhammer CM, Blankschtein D, Langer R. Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expert Opin Drug Deliv. 2014;11:393–407.
Vitorino C, Sousa J, Pais A. Overcoming the skin permeation barrier: challenges and opportunities, Curr Pharm Des, (2015) 2698–2712.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2012;64:128–37.
Pathan IB, Setty CM. Chemical penetration enhancers for transdermal drug delivery systems. Trop J Pharm Res. 2009;8:173–9.
Ahad A, Kohli C, Sultana MT. Chemical penetration enhancers: a patent review. Expert Opin. Ther. Patents. 2009;19:969–88.
Lopes LB, Garcia MT, Bentley MV. Chemical penetration enhancers. Ther Deliv. 2015;6:1053–61.
Haque T, Talukder MMU. Chemical enhancer: a simplistic way to modulate barrier function of the stratum Corneum. Adv Pharm Bull. 2018;8:169–79.
Hsu T, Mitragotri S. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. Proc Natl Acad Sci U S A. 2011;108:15816–21.
Sunny K, Michael Z. M. Chen., peptides as skin penetration enhancers: mechanisms of action. J Control Release. 2014;199:168–78.
Raphael AP, Primiero CA, Ansaldo AB, Keates HL, Soyer HP, Prow TW. Elongate microparticles for enhanced drug delivery to ex vivo and in vivo pig skin. J Control Release. 2013;172:96–104.
Zhang C, Zhang K, Zhang J, Ou H, Duan J, Zhang S, et al. Skin delivery of hyaluronic acid by the combined use of sponge spicules and flexible liposomes. Biomater Sci. 2019;7:1299–310.
Karande P, Jain A, Mitragotri S. Relationships between skin's electrical impedance and permeability in the presence of chemical enhancers. J Control Release. 2006;110:307–13.
Zhang S, Ou H, Liu C, Zhang Y, Mitragotri S, Wang D, et al. Skin delivery of hydrophilic biomacromolecules using marine sponge spicules. Mol Pharm. 2017;14:3188–200.
Chen M, Gupta V, Anselmo AC, Muraski JA, Mitragotri S. Topical delivery of hyaluronic acid into skin using SPACE-peptide carriers. J Control Release. 2014;173:67–74.
Chen M, Kumar S, Anselmo AC, Gupta V, Slee DH, Muraski JA, et al. Topical delivery of cyclosporine a into the skin using SPACE-peptide. J Control Release. 2015;199:190–7.
Laffleur F, Pschick S, Barthelmes J, Hauptstein S, Bernkop-Schnurch A. Impact of surfactants on skin penetration of Dexpanthenol. Current Drug Delivery. 2018;15:351–6.
Wen MM, El-Kamel AH, Khalil SA. Systemic enhancement of papaverine transdermal gel for erectile dysfunction. Drug Dev Ind Pharm. 2012;38:912–22.
Limpongsa E, Umprayn K. Preparation and evaluation of diltiazem hydrochloride diffusion-controlled transdermal delivery system. AAPS PharmSciTech. 2008;9:464–70.
Karande P, Jain A, Mitragotri S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat Biotechnol. 2004;22:192–7.
Davies DJ, Ward RJ, Heylings JR. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol in Vitro. 2004;18:351–8.
Peck KD, Ghanem A-H, Higuchi WI. Hindered diffusion of polar molecules through and effective pore radii estimates of intact and ethanol treated human epidermal membrane. Pharm Res. 1994;11:1306–14.
Li SK, Ghanem AH, Peck KD, Higuchi WI. Pore induction in human epidermal membrane during low to moderate voltage iontophoresis: a study using AC iontophoresis. J Pharm Sci-Us. 1999;88:419–27.
Tezel A, Sens A, Mitragotri S. Description of transdermal transport of hydrophilic solutes during low-frequency sonophoresis based on a modified porous pathway model. J Pharm Sci. 2003;92:381–93.
Cevc G, Vierl U. Nanotechnology and the transdermal route: a state of the art review and critical appraisal. J Control Release. 2010;141:277–99.
Tezel A, Sens A, Mitragotri S. A theoretical analysis of low-frequency sonophoresis: dependence of transdermal transport pathways on frequency and energy density. Pharm Res. 2002;19:1841–6.
Mitragotri S, Anissimov YG, Bunge AL, Frasch HF, Guy RH, Hadgraft J, et al. Mathematical models of skin permeability: an overview. Int J Pharm. 2011;418:115–29.
Schatzlein A, Cevc G. Non-uniform cellular packing of the stratum corneum and permeability barrier function of intact skin: a high-resolution confocal laser scanning microscopy study using highly deformable vesicles (Transfersomes). Br J Dermatol. 1998;138:583–92.
Notman R, Anwar J, Briels WJ, Noro MG, den Otter WK. Simulations of skin barrier function: free energies of hydrophobic and hydrophilic Transmembrane pores in Ceramide bilayers. Biophys J. 2008;95:4763–71.
Gowrishankar TR, Weaver JC. Electrical behavior and pore accumulation in a multicellular model for conventional and supra-electroporation. Biochem Biophys Res Commun. 2006;349:643–53.
Polat BE, Seto JE, Blankschtein D, Langer R. Application of the aqueous porous pathway model to quantify the effect of sodium lauryl sulfate on ultrasound-induced skin structural perturbation. J Pharm Sci. 2011;100:1387–97.
Schepens B, Vos PJ, Saelens X, van der Maaden K. Vaccination with influenza hemagglutinin-loaded ceramic nanoporous microneedle arrays induces protective immune responses. Eur J Pharm Biopharm. 2019;136:259–66.
Vallhov H, Xia W, Engqvist H, Scheynius A. Bioceramic microneedle arrays are able to deliver OVA to dendritic cells in human skin. J Mater Chem B. 2018;6:6808–16.
Jung SJ, Choi SO, Um SY, Kim JI, Choo HYP, Choi SY, et al. Prediction of the permeability of drugs through study on quantitative structure-permeability relationship. J Pharm Biomed Anal. 2006;41:469–75.
Tsakovska I, Pajeva I, Al Sharif M, Alov P, Fioravanzo E, Kovarich S, et al. Quantitative structure-skin permeability relationships. Toxicology. 2017;387:27–42.
Moss GP, Dearden JC, Patel H, Cronin MTD. Quantitative structure-permeability relationships (QSPRs) for percutaneous absorption. Toxicol in Vitro. 2002;16:299–317.
Oh SY, Leung L, Bommannan D, Guy RH, Potts RO. Effect of current. Ionic-Strength and Temperature on the Electrical-Properties of Skin, Journal of Controlled Release. 1993;27:115–25.
Karande P, Mitragotri S. High throughput screening of transdermal formulations. Pharm Res. 2002;19:655–60.
Acknowledgements and Disclosures
This work was supported in part by Science and Technology Planning Project of Fujian Province, China (Grant No. 2017Y4015), Marine Economic Development Subsidy Project of Fujian Province, China(Grant No. FJHJF-L-2019-03), Medical Elite Cultivation Program of Fujian Province, China (Grant No. 2015-ZQN-ZD-24). M.C. designed the experiments. X.L., Z.W. and H.O. performed the experiments. X.L. and M.C. wrote the article with the help of S.M.. All the authors were involved in the analyses and interpretation of data. We thank Yuhao Lu for assistance with spicule purification, Saiman Zhang for assistance with the preparation of porcine skin. There are no conflicts to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, X., Wang, Z., Ou, H. et al. Correlations Between Skin Barrier Integrity and Delivery of Hydrophilic Molecules in the Presence of Penetration Enhancers. Pharm Res 37, 100 (2020). https://doi.org/10.1007/s11095-020-02800-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02800-4