Abstract
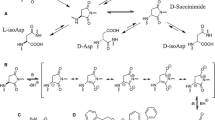
The influence of the primary sequence on the degradation of Asp4 residues (e.g., formation of the cyclic imide and Asp-X and/or X-Asp amide bond hydrolysis) was investigated using Val-Tyr-Y-Asp-X-Ala hexapeptides. These reactions were proposed to involve cyclization, which would duly be sensitive to steric hindrance. The effects on the rates of individual degradation routes and product distribution under both acidic and alkaline conditions were assessed upon substitutions made on the C-terminal side (X) and on the N-terminal side (Y) of the Asp residue. As expected, the rate of intramolecular formation of cyclic imide and, thus, the product yield were most affected by the size of the amino acid on the C-terminal side of the Asp residue. However, such structural changes had little or no impact on the rate of Asp-X and Y-Asp amide bond hydrolysis. In the former case, the substituted site was one atom removed from the reaction site, accounting for the diminished steric effect observed. As for the latter, the site of substitution was not a participant in the reaction itself, and hence, the rate was unperturbed by this modification. Placing Ser and Val C terminally to the Asp residue prompted racemization and peptide bond hydrolysis to occur under alkaline conditions. N-Terminal substitution of Pro with Gly had no effect on the rate of isomerization via cyclic imide formation but greatly enhanced the rate of Y-Asp amide bond hydrolysis.
Similar content being viewed by others
REFERENCES
M. C. Manning, K. Patel, and R. T. Borchardt. Stability of protein pharmaceuticals. Pharm. Res. 6:903–918 (1989).
A. Sun, K. U. Yuksel, and R. W. Gracy. Relationship between the catalytic center and the primary degradation site of triose-phosphate isomerase: Effects of active site modification and deamidation. Arch. Biochem. Biophys. 293:382–390 (1992).
I. M. Ota and S. Clarke. Calcium affects the spontaneous degradation of aspartyl/asparaginyl residues in calmodulin. Biochemistry 28:4020–4027 (1989).
U. J. Lewis, E. V. Cheever, and W. C. Hopkins. Kinetic study of the deamidation of growth hormone and prolactin. Biochim. Biophys. Acta 214:498–508 (1970).
K. Patel and R. T. Borchardt. Chemical pathways of peptide degradation. II. Kinetics of deamidation of an asparaginyl residue in a model hexapeptide. Pharm. Res. 7:787–793 (1990).
R. C. Stephenson and S. Clarke. Succinimide formation in aspartyl and asparaginyl-containing peptides as a model for the degradation of proteins. J. Biol. Chem. 264:6164–6170 (1989).
S. Capasso, L. Mazzarella, F. Sica, and A. Zagani. Deamidation via cyclic imide in asparaginyl peptides. Peptide Res 2:195–200 (1989).
T. Geiger and S. Clarke. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides: Succinimide-linked reactions that contribute to protein degradation. J. Biol. Chem. 262:785–794 (1987).
M. Bodanszky and J. Z. Kwei. Side reactions in peptide synthesis. Int. J. Peptide Protein Res. 12:69–74 (1978).
T. Baba, H. Sugiyama, and S. Seto. Rearrangement of α-to β-aspartyl peptide with anhydrous hydrogen fluoride. Chem. Pharm. Bull. 21:207–209 (1973).
Y. Shalitin and S. A. Bernhard. Co-operative effects of functional groups in peptides. II. Elimination reactions in aspartyl-(O-acyl)-serine derivatives. J. Am. Chem. Soc. 88:4711–4721 (1966).
F. Marcus. Preferential cleavage at aspartyl-prolyl peptide bonds in dilute acid. Int. J. Peptide Protein Res. 25:542–546 (1985).
M. Landon. Cleavage at aspartyl-prolyl bonds. Methods Enzymol. 47:145–149 (1977).
D. Piszkiewicz, M. Landon, and E. L. Smith. Anomalous cleavage of aspartyl-proline peptide bonds during amino acid sequence determinations. Biochem. Biophys. Res. Commun. 40:1173–1178 (1970).
C. Oliyai and R. T. Borchardt. Chemical pathways of peptide degradation. IV. Pathways, kinetics, and mechanism of degradation of an aspartyl residue in a model hexapeptide. Pharm. Res. 10:95–102 (1993).
G. Odian. Principles of Polymerization, 2nd ed., Wiley-Interscience, New York, 1970, p. 80.
A. S. Inglis. Cleavage at aspartic acid. Methods Enzymol. 91:324–332 (1983).
K. Iwai and T. Ando. N,O acyl rearrangement. Methods Enzymol. 11:263–282 (1967).
L. V. Pavlova and F. Y. Rachinskii. Rearrangements connected with the migration of acyl and certain other groups. Russ. Chem. Rev. 37:587–602 (1968).
S. A. Bernhard, A. Berger, J. H. Carter, E. Katchalski, M. Sela, and Y. Shalitin. Co-operative effects of functional groups in peptides. I. Aspartyl-serine derivatives. J. Am. Chem. Soc. 84:2421–2435 (1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oliyai, C., Borchardt, R.T. Chemical Pathways of Peptide Degradation. VI. Effect of the Primary Sequence on the Pathways of Degradation of Aspartyl Residues in Model Hexapeptides. Pharm Res 11, 751–758 (1994). https://doi.org/10.1023/A:1018944800691
Issue Date:
DOI: https://doi.org/10.1023/A:1018944800691