Abstract
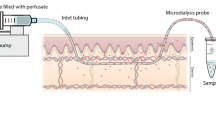
Microdialysis sampling of the dermis in vivo was accomplished using a linear microdialysis probe. In contrast to previous studies using a commercial cannula-style microdialysis probe, the linear probe had no effect on the flux of drug through the skin in vitro. The extent of tissue damage in vivo due to probe implantation was evaluated by histological examination and microdialysis delivery studies. Tissue damage due to implantation of the linear probe was minimal with no bleeding or edema observed. Infiltration of lymphocytes into the tissue was observed beginning 6 hours after probe implantation with scar tissue beginning to form after approximately 32 hours. The infiltration of lymphocytes had no effect on the behavior of implanted microdialysis probes. Delivery of 5-fluorouracil was between 20 and 25% for six different probes implanted in six different animals demonstrating good probe-to-probe and implantation-to-implantation reproducibility. Constant delivery was maintained for at least 24 hours in all cases indicating that experiments of at least 24 hour duration are feasible. The dermal concentration of topically applied 5-FU cream, Efudex®, was continuously monitored by an implanted microdialysis probe demonstrating the feasibility of this technique as for monitoring skin drug levels in vivo. The dermal concentration of 5-FU following topical application was approximately 40-fold higher for in vitro excised skin than for in vivo intact skin.
Similar content being viewed by others
REFERENCES
S. Chong and H.-L. Fung. Transdermal Drug Delivery Systems: Pharmacokinetics, Clinical Efficacy, and Tolerance Development, in “Transdermal Drug Delivery” (J. Hadgraft and R.H. Guy, eds.) Marcel Dekker: New York, 1989, pg. 135–153.
R.H. Guy and J. Hadgraft. Transdermal Drug Delivery — A Simplified Pharmacokinetic Approach. Int. J. Pharm. 24:267–274(1985).
R.H. Guy, J. Hadgraft and D.A.W. Bucks. Transdermal Drug Delivery and Cutaneous Metabolism. Xenobiotica 17:325–343(1987).
J.W. Stone, J.M. Andrews, J.P. Ashby, D. Griggs, and R. Wise. Pharmacokinetics and Tissue Penetration of Orally Administered Lomefloxacin. Antimicrob. Agents Chemother. 32:1508–1510(1988).
R.L. Bronaugh and H.I. Maibach. In Vitro Models for Human Percutaneous Adsorption, in “Models in Dermatology” (H.I. Maibach and N.J. Lowe, eds.) Karger: Basel, 1985, p. 178–188.
Y.W. Chien. Development of Transdermal Drug Delivery Systems. Drug Dev. Ind. Pharm. 13:589–651(1987).
R.H. Guy, J. Hadgraft, R.S. Hinz, K.V. Roskos and D.A.W. Bucks, in “Transdermal Controlled Systemic Medications” (Y.W. Chien, ed.), Marcel Dekker: New York, 1987, pg. 205.
J. M. Ault, C. E. Lunte, N. M. Meltzer, and C. M. Riley. Microdialysis sampling for the investigation of dermal drug transport. Pharm. Res. 9:1256–1261 (1992).
U. Ungerstedt and C. Pycock. Functional correlates of dopamine neurotransmission. Bull. Schweiz. Akad. Med. Wiss. 1278:1–13 (1974).
N. W. Bunnett, M. Mogard, M. S. Orloff, H. J. Corbet, J. R. Reeve, Jr., and J. H. Walsh. Catabolism of neurotensin in interstitial fluid of the rat stomach. Am. J. Physiol. 246:675–682 (1984).
U. Tossman, T. Wieloch, and U. Ungerstedt. Gama-amino-butyric acid and taurine release in the striatum of the rat during hypoglycemic coma studied by microdialysis. Neurosci. Lett. 62:231–235 (1985).
H. Jarry, E. M. Duher, and W. Wuttke. Adrenal release of catecholamine and metenkephalin before and after stress as measured by a novel in vivo dialysis method on the rat. Neurosci. Lett. 60:273–278 (1985).
A. Imperato and G. Di Chiara. Trans-striatal dialysis coupled to reverse phase high performance liquid chromatography with electrochemical detection: a new method for the study of the in vivo release of endogenous dopamine metabolites. J. Neurosci. Lett. 4:966–977 (1984).
K. Matsuyama, M. Nakashima, Y. Nakaboh, M. Ichikawa, T. Yano, and S. Satoh. Application of in vivo microdialysis to transdermal absorption of methotrexate in rats. Pharm. Res. 11:684–686(1994).
E. V. Hagstrom-Toft, P. Arner, U. Johansson, L. S. Eriksson, U. Ungerstedt and J. Bolinder. Effect of insulin on human adipose tissue metabolism in situ. Interactions with beta-adrenoceptors. Diabetologia 35:664–670 (1992).
D. Scheller and J. Kolb. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialyzate samples. J. Neurosci. Methods 40:31–38 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ault, J.M., Riley, C.M., Meltzer, N.M. et al. Dermal Microdialysis Sampling in Vivo . Pharm Res 11, 1631–1639 (1994). https://doi.org/10.1023/A:1018922123774
Issue Date:
DOI: https://doi.org/10.1023/A:1018922123774