Abstract
Purpose. To determine if intestinal secretion occurs for the poorly bioavailable diuretic, furosemide.
Methods. Jejunal segments of male Sprague-Dawley rats were mounted on diffusion chambers, and the permeation of furosemide was measured across the excised tissue in both directions. Studies were repeated using cultured epithelia from adenocarcinoma cells (Caco-2) grown on filter inserts mounted in 6-well plates. Temperature-dependence and chemical inhibition by indomethacin was also tested using the cell culture model.
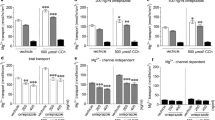
Results. Net secretion from rat intestine of over 3-fold was observed for 20 µM furosemide. Net secretion of furosemide by Caco-2 cells was over 300% greater than for intestinal segments (10-fold vs. 3-fold). For both models, a decrease in furosemide transport in the direction of secretion was observed in the presence of indomethacin (100 µM), although only results using the Caco-2 cells showed an increase in the absorptive transport. Furosemide secretion from Caco-2 cells decreased with decrease in temperature from 37°C to 4°C, suggesting a carrier-mediated process.
Conclusions. Furosemide appears to be secreted in the small intestine. These preliminary results indicate that furosemide bioavailability may be limited by an intestinal transporter.
Similar content being viewed by others
REFERENCES
E. Jackson. Diuretics. In Goodman and Gilman's: The Pharmacological Basis of Therapeutics, 9th Edition, J. Hardman, L. Limbird, P. Molinoff, R. Ruddon, and A. G. Gilman (eds.), McGraw-Hill, New York, 1996, pp. 685–713.
M. Hammarlund-Udenaes and L. Z. Benet. Furosemide pharmacokinetics and pharmacodynamics in health and disease—An update. J. Pharmacokin. Biopharm. 17:1–46 (1989).
M. R. Kelly, R. E. Cutler, A. W. Forrey, and B. M. Kimpel. Pharmacokinetics of orally administered furosemide. Clin. Pharmacol. Ther. 15:178–86 (1974).
H. Bundgaard, T. Noørgaard, and N. M. Nielsen. Photodegradation and hydrolysis of furosemide and furosemide esters in aqueous solutions. Int. J. Pharm. 42:217–24 (1988).
R. K. Verbeeck, R. V. Patwardhan, J.-P. Villeneuve, G. R. Wilkinson, and R. A. Branch. Furosemide disposition in cirrhosis. Clin. Pharmacol. Ther. 31:719–25 (1982).
M. G. Lee and W. L. Chiou. Evaluation of potential causes for the incomplete bioavailability of furosemide: gastric first-pass metabolism. J. Pharmacokin. Biopharm. 11:623–40 (1983).
J. Valentine, D. C. Brater, and G. J. Krejs. Clearance of furosemide by the gastrointestinal tract. J. Pharmacol. Exp. Ther. 236:177–80 (1986).
D. E. Smith and L. Z. Benet. Biotransformation of furosemide in kidney transplant patients. Eur. J. Clin. Pharmacol. 24:787–90 (1983).
V. Pichette and P. du Soich. Role of the kidneys in the metabolism of furosemide: Its inhibition by probenecid. J. Am. Soc. Nephrol. 7:345–9 (1996).
M. F. Paine, D. D. Shen, K. L. Kunze, J. D. Perkins, C. L. Marsh, J. P. McVicar, D. M. Barr, B. S. Gillies, and K. E. Thummel. First-pass metabolism of midazolam by the human intestine. Clin. Pharmacol. Ther. 60:14–24 (1996).
U. Mayer, E. Wagenaar, J. H. Beijnen, J. W. Smit, D. K. F. Meijer, J. van Aspereren, P. Borst, and A. Schinkel. Substantial excretion of digoxin via the intestinal mucosa and prevention of long-term digoxin accumulation in the brain by the mdrla P-glycoprotein. Br. J. Pharmacol. 119:1038–44 (1996).
C. Y. Wu, L. Z. Benet, M. F. Hebert, S. K. Gupta, M. Rowland, D. Y. Gomez, and V. J. Wacher. Differentiation of absorption and first-pass gut and hepatic metabolism in humans: Studies with cyclosporine. Clin. Pharmacol. Ther. 58:492–7 (1995).
F. Thiebaut, T. Tsuro, H. Hamada, M. M. Gottesman, I. Pastan, and M. C. Willingham. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissue. Proc. Nat. Acad. Sci., USA 84:7735–38 (1987).
P. Watkins. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Adv. Drug Del. Rev. 27:161–70 (1997).
D. E. Smith, W. L. Gee, D. C. Brater, E. Lin, and L. Z. Benet. Preliminary evaluation of furosemide-probenecid interaction in humans. J. Pharm. Sci. 69:571–5 (1980).
H. Saitoh and B. J. Aungst. Possible involvement of multiple P-glycoprotein-mediated efflux systems in the transport of verapamil and other organic cations across rat intestine. Pharm. Res. 12:1304–10 (1995).
I. J. Hidalgo, T. J. Raub, and R. T. Borchardt. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–49 (1989).
A. Hilgers, R. A. Conradi, and P. S. Burton. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm. Res. 7:902–10 (1990).
F. Delie and W. Rubas. A human colonic cell line sharing similarities with eneterocytes as a model to examine oral absorption: Advantages and limitations of the Caco-2 model. Crit. Rev. Therap. Drug Carrier Sys. 14:221–85 (1997).
D. E. Smith, D. C. Brater, E. Lin, and L. Z. Benet. Attenuation of furosemide's pharmacokinetic effect by indomethacin: Pharmacokinetic evaluation. J. Pharmacokin. Biopharm. 7:265–74 (1979).
R. Bendayan. Renal drug transport: A review. Pharmacotherapy 16:971–85 (1996).
G. K. Collington, J. Hunter, C. N. Allen, N. L. Simmons, and B. H. Hirst. Polarized efflux of 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein from cultured epithelial cell monolayers. Biochem. Pharmacol. 44:417–24 (1992).
H. Saitoh, C. Gerard, and B. J. Aungst. The secretory intestinal transport of some beta-lactam antibiotics and anionic compounds: A mechanism contributing to poor oral absorption. J. Pharmacol. Exp. Ther. 278:205–11 (1996).
M. P. Draper, R. L. Martell, and S. B. Levy. Indomethacin-mediated reversal of multidrug reistance and drug efflux in human and murine cell lines overexpressing MRP, but not P-glycoprotein. Br. J. Cancer 75:810–15 (1997).
M. Kool, M. de Haas, G. L. Scheffer, R. J. Scheper, M. van Eijk, J. A. Juijn, F. Baas, and P. Borst. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP-5, homologues of the multidrug resistance-associated protein gene (MRP-1), in human cancer cell lines. Cancer Res. 57:3537–47 (1997).
Z. Holló, L. Homolya, T. Hegedüs, and B. Sarkadi. Transport properties of the multidrug resistance-associated protein (MRP) in human tumour cells. FEBS Lett. 383:99–104 (1996).
R. Evers, G. Zaman, L. van Deetmer, H. Jansen, J. Calafat, L. Oomen, R. Oude Elferink, P. Borst, and A. H. Schinkel. Basolateral localization and export activity of the human multidrug resistance-associated protein in polarized pig kidney cells. J. Clin. Invest. 97:1211–18 (1996).
R. Evers, M. Kool, L. van Deetmer, H. Jansen, J. Calafat, L. Oomen, C. C. Paulusma, R. Oude Elferink, F. Baas, A. H. Schinkel, and P. Borst. Drug export activity of the human canicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J. Clin. Invest. 101:1310–19 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flanagan, S.D., Benet, L.Z. Net Secretion of Furosemide Is Subject to Indomethacin Inhibition, as Observed in Caco-2 Monolayers and Excised Rat Jejunum. Pharm Res 16, 221–224 (1999). https://doi.org/10.1023/A:1018868123367
Issue Date:
DOI: https://doi.org/10.1023/A:1018868123367