Abstract
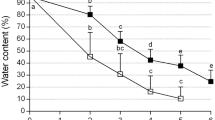
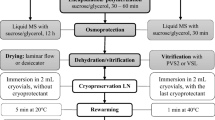
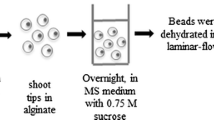
Alginate-coated meristems from in vitro-grown strawberry (Fragaria × ananassa Duch.) were successfully cryopreserved following dehydration by a vitrification solution. Excised meristems from cold-hardened plantlets at 4 °C for two weeks in the dark were encapsulated into alginate-gel beads containing a mixture of 2 M glycerol plus 0.4 M sucrose. These encapsulated and cryoprotected meristems were dehydrated with a highly concentrated vitrification solution (designated PVS2) for 2 hr at 0 °C prior to a plunge into liquid nitrogen. Successfully encapsulated vitrified meristems remained green and then developed shoots within one week after plating without intermediary callus formation. The average rate of shoot formation of encapsulated vitrified meristems amounted to nearly 90%. The cryogenic protocol was successfully applied to four cultivars of strawberry. It was also confirmed that encapsulated vitrified meristems cooled to - 196 °C produced higher shoot formation than encapsulated dried meristems. Besides, the recovery growth was much earlier than the latter. The encapsulation-vitrification method is easy to handle and produces high levels of shoot formation. Thus, the protocol promises for cryopreservation of strawberry germplasm.
Similar content being viewed by others
References
Bachiri, Y., C. Gazeau, J. Hansz, C. Morisset & J. Dereuddre, 1995. Successful cryopreservation of suspension cells by encapsulationdehydration. Plant Cell Tiss Org Cult 43: 241- 248.
Benson, E.E., B.M. Reed, R.M. Brennam, K.A. Clacher & D.A. Ross, 1996. Use of thermal analysis in the evaluation of cryopreservation protocols for Ribes nigrumL. germplasm. CryoLetters 17: 347-362.
Dereuddre, J., J. Fabre & C. Bassaglia, 1988. Resistance to freezing in liquid nitrogen of carnation (Dianthus caryophyllusL. var. Kolo) apical and axially shoot tips excised from different aged in vitroplantlets. Plant Cell Rep 7: 170-173.
Fabre, J. & J. Dereuddre, 1990. Encapsulationdehydration: a new approach to cryopreservation of Solanumshoottips. CryoLetters 11: 413-426.
Fukai, S., M. Goi & M. Tanaka, 1991. Cryopreservation of shoot tips of Chrysanthemum morifoliumand related species native to Japan. Euphytica 54: 201-204.
González-Arnao, M.T., T. Moreira & C. Urra, 1996. Importance of pregrowth with sucrose and vitrification for the cryopreservation on sugarcane apices using encapsulationdehydration. CryoLetters 17: 141-148.
Haskins, R.H. & K.K. Kartha, 1980. Freeze preservation of pea meristems: cell survival. Can J Bot 58: 833-839.
Kartha, K.K., L. Leung & K. Pahl, 1980. Cryopreservation of strawberry meristems and mass propagation of plantlets. J Am Soc Hort Sci 105(4): 481-484.
Kuranuki, Y. & A. Sakai, 1995. Cryopreservation of in vitro-grown shoot tips of tea (Camellia sinensis) by vitrification. CryoLetters 16: 345-352.
Langis, R., B.J. Schnabel-Preikstas, E.D. Earle & P.H. Steponkus, 1990. Cryopreservation of in vitro-grownshoot tips by vitrification. Cryobiol 27: 657-658.
Matsumoto, T., A. Sakai & K. Yamada, 1994. Cryopreservation of in vitro-grownapical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep 13: 442-446.
Matsumoto, T., A. Sakai, C. Takahashi & K. Yamada, 1995a. Cryopreservation of in vitro-grownapical meristems of wasabi (Wasabia japonica) by encapsulation-vitrification method. CryoLetters 16: 189-196.
Matsumoto, T., A. Sakai & K. Yamada, 1995b. Cryopreservation of in vitro-grownapical meristems of lily by vitrification. Plant Cell Tiss Org Cult 41: 237-241.
Mullin, R.H. & D.E. Schlegel, 1976. Cold storage maintenance of strawberry meristem plantlets. Hortsci 11(2): 192-194.
Murashige, T. & F. Skoog, 1962. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15: 473- 497.
Niino, T. & A. Sakai, 1992a. Cryopreservation of alginatecoated in vitro-grownshoot tips of apple, pear, and mulberry. Plant Sci 87: 199-206.
Niino, T., A. Sakai, S. Enomoto, J. Magoshi & S. Kato, 1992b. Cryopreservation of in vitro-grownshoot tips of mulberry by vitrification. Cryo-Letters 13: 303-312.
Niino, T., A. Sakai, H. Yakuwa & K. Nojiri, 1992c. Cryopreservation of in vitro-grownshoot tips of apple and pear by vitrification. Plant Cell Tiss Org Cult 28: 261-266.
Nishizawa, S., A. Sakai, Y. Amano & T. Matsuzawa, 1993. Cryopreservation of asparagus (Asparagus officinalisL.) embryogenic suspension cells and subsequent plant regeneration by vitrification method. Plant Sci 88: 67-73.
Reed, B.M., 1990. Survival of in vitro-grownapical meristems of Pyrusfollowing cryopreservation. HortSci 25: 111-113.
Reed, B.M. & K.E. Hummer, 1995. Conservation of germplasm of strawberry. In: Y.P.S. Bajaj (Ed.), Biotechnology in Agriculture and Forestry, Vol. 32, pp. 354-370. SpringerVerlag, Berlin.
Reinhoud, P., 1996. Cryopreservation of tobacco suspension cells by vitrification. In: Doctoral Paper, pp. 1-95. Rijks Universiteit, Leiden.
Sakai, A., 1995. Cyropreservation of germplasm of woody plants. In: Y.P.S. Bajaj (Ed.), Biotechnology in Agriculture and Forestry, Vol. 52, pp. 53-69. SpringerVerlag, Berlin.
Sakai, A., M. Yamakawa, D. Sakata, T. Harada & T. Yakuwa, 1978. Development of a whole plant from an excised strawberry runner apex frozen to 196 °C. Low Temp Sci Ser B36: 31-38.
Sakai, A., S. Kobayashi & I. Oiyama, 1990. Cryopreservation of nucellar cells of naval orange (Citrus sinesisOsb. var. brasiliensisTanaka) by vitrification. Plant Cell Rep 9: 30-33.
Takagi, H., N.T. Thinh, O.M. Isulam, T. Senboku & A. Sakai, 1997. Cryopreservation of in vitro-grownshoot tips of taro (Colocasia esculenta(L.) Schott) by vitrification. 1. Investigation of basic conditions of the vitrification procedure. Plant Cell Rep 16: 594- 599.
Tannoury, M., J. Ralambosoa, M. Kaminski & J. Dereuddre, 1991. Cryopreservation by vitrification of alginate-coated carnation (Dianthus caryophyllusL.) shoot tips of in vitroplantlets. C R Acad Sci Paris, t. 313, Série III: 633-638.
Thinh, N.T., 1997. Cryopreservation of germplasm of vegetatively propagated tropical monocots by vitrification. Doctoral Thesis, Dept of Agronomy, Kobe University (in press).
Touchell, D.H., 1995. Principles of cryobiology for conservation of threatened Australian plants. Doctoral Thesis, Dept of Botany, Western University, Perth.
Touchell, D.H. & K.W. Dixson, 1995. Cryopreservation for the conservation of Australian endangered plants. p. 33. Int Workshop on the In VitroConservation of Plant Genetic Resources (organized by University Kebangsaan Malaysia and IBPGRI), Kuala Lumpur.
Towill, L.E., 1984. Survival of ultra-low temperatures of shoottips from Solanum tuberosumgroups Andigena, Phureja, Stenotomum and other tuberbearing Solanumspecies. CryoLetters 5: 319-326.
Towill, L.E., 1990. Cryopreservation of isolated mint shoot tips by vitrification. Plant Cell Rep 9: 178-180.
Uragami, A., A. Sakai & M. Nagai, 1990. Cryopreservation of dried axially buds from plantlets of Asparagus officinalisL. grown in vitro. Plant Cell Rep 9: 328-331.
Widholm, J.M., 1972. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cell. Stain Technol 47: 189-194.
Yamada, T., A. Sakai, T. Matsumura & S. Higuchi, 1991. Cryopreservation of apical meristems of white clover (Trifolium repensL.) by vitrification. Plant Sci 78: 81-87.
Yamada, T., A. Sakai, T. Matsumura & S. Higuchi, 1993. Plant regeneration of meristematic callus of white clover (Trifolium repensL.) cooled to 196-°C by vitrification. Euphytica 70: 197-203.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hirai, D., Shirai, K., Shirai, S. et al. Cryopreservation of in vitro-grown meristems of strawberry (Fragaria × ananassa Duch.) by encapsulation-vitrification. Euphytica 101, 109–115 (1998). https://doi.org/10.1023/A:1018386211577
Issue Date:
DOI: https://doi.org/10.1023/A:1018386211577