Abstract
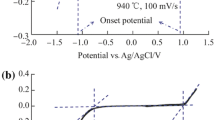
The anodic behaviour of compacted graphite, graphite powder, glassy carbon and reticulated vitreous carbon electrodes in basic sodium chloroaluminate melt in the temperature range 428–573 K was studied using cyclic voltammetry. Chlorine evolution (> + 2.1 V vs Al) alone was the predominant reaction on the compact glassy carbon and fresh RVC electrodes. On compacted graphite, chlorine-assisted chloroaluminate intercalation was found to be a competitive process to the chlorine evolution. At high sweep rates, intercalation/deintercalation near the graphite lattice edges occur faster than chlorine evolution. Subsequent intercalation, however, is a slow process. Chlorine evolution predominates at higher temperatures and at higher anodic potentials. On graphite powders, a more reversible free radical chlorine adsorption/desorption process also occurs in the potential region below chlorine evolution. The process occurs at the grain boundaries, edges and defects of the graphite powder material. Intercalation/deintercalation processes are mainly responsible for the disintegration of graphitic materials in low-temperature chloroaluminate melts. Repeated intercalation/deintercalation cycles result in the irreversible transformation of the electrode surface and electrode characteristics. The surface area of the electrode is increased substantially on cycling. Electrode materials and operating conditions suitable for chlorine generation, intercalation/deintercalation and chlorine adsorption/desorption and power sources based on these processes are identified in this work.
Similar content being viewed by others
References
British Patent, 1 200 103 (1967).
Yashuhiko Ito and Shiro Yoshizawa, Some new molten salt electrolytic processes, in G. Mamantov and J. Braunstein (Eds), 'Advances in Molten Salt Chemistry', (Plenum, New York, 1981), p.391.
K.S. Mohandas, N. Sanil and P. Rodriguez, Proceedings of the National Symposium on Electrochemistry in Nuclear Technology (Kalpakkam, 1998), pp. 163–168.
H. Wendt, A. Khalil and C.E. Padberg, J. Appl. Electrochem. 21 (1991) 929.
G.L. Holleck, J. Electrochem. Soc. 119 (1972) 1158.
A.J. Bard, 'Encyclopedia of Electrochemistry of the Elements' (Marcel Dekker, Inc., New York, 1976) vol. X, p. 263.
H. Wendt, S. Dermeik and A. Ziogas, Werkst Korros. 41 (1990) 457–463.
K.S. Mohandas, PhD thesis, University of Madras, January (2001).
S. Maximovitch, M. Levart, M. Fouletier, N. Nguyen and G. Bronoel, J. Power Sources 3 (1978) 215–225.
K.S. Mohandas, N. Sanil, Tom Mathews and P. Rodriguez, Metall. Mater. Trans. B, in press.
W. Rudorff, in 'Advances in Inorganic Chemistry and Radiochemistry' vol. 1, (1959), p. 254.
W. Rudorff and A. Landel, Z. Anorg. Allg. Chem. 293 (1958) 327.
M.L. Dzurus and G.R. Henning, J. Amer. Chem. Soc. 79 (1957) 1051.
B. Bach and A.R. Ubbelhode, Proc. R. Soc. Lond. A 325 (1971) 437.
J.G. Hooley, Carbon 11 (1973) 225.
R.C. Croft, J. Appl. Chem. (Lond.) 2 (1952) 557.
J.G. Hooley and P.T. Hough, Carbon 16 (1978) 221.
Baikar, E. Habegger, V.K. Sharma and W. Richard, Carbon 19 (1981) 329.
Baikar, E. Habegger and R. Schlogl, Ber. Bunsenges. Phys. Chem. 89 (1985) 530.
M. Fouletier and M. Armand, Carbon 17 (1979) 427.
J.G. Hooley, Carbon 18 (1980) 83.
E. Stumpp, Mater. Sci. Eng. 31 (1977) 53.
D.K. Gosser, Jr, 'Cyclic Voltammetry — Simulation and Analysis of Reaction Mechanisms' (VCH, New York, 1996), p. 43.
H. Thiele, Z. Electrochem. 40 (1934) 26.
K. Kinoshita, 'Carbon: Electrochemical and Physiochemical Properties' (J. Wiley & Sons, New York, 1988).
J.J. Werth, US Patent 3 847 667 (1974).
P. Beck, H. Junge and H. Krohn, Electrochim. Acta 26 (1981) 799.
L.J.J. Janssen and J.G. Hoogland, Electrochim. Acta 15 (1970) 941.
L.J.J. Janssen, Electrochim. Acta 19 (1974) 257.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mohandas, K., Sanil, N., Noel, M. et al. Anodic behaviour of carbon materials in NaCl saturated NaAlCl4 fused electrolyte at low temperatures: A cyclic voltammetric study. Journal of Applied Electrochemistry 31, 997–1007 (2001). https://doi.org/10.1023/A:1017966316057
Issue Date:
DOI: https://doi.org/10.1023/A:1017966316057