Abstract
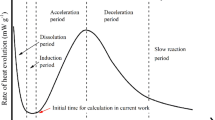
After the initial mixing of cement, an induction period occurs during which its consistency remains constant. Thickening occurs at the end of this period when the consistency is observed to increase very rapidly. In this paper we propose a reaction-diffusion model for the hydration of tricalcium silicate, a principal constituent of cement, which is believed to be responsible for the initial development of its strength. Our model is based on the assumption that the hydration of cement can be described as a dissolution -precipitation reaction. The mathematical solutions enable us to determine some of the factors that control the length of the induction period and make predictions of the ionic concentrations which are in agreement with experimental data.
Similar content being viewed by others
References
J. D. Birchall, A. J. Howard and J. E. Bailey. On the Hydration of Portland Cement. Proc. R. Soc. London 360 (1978) 445–453.
D. D. Double. New developments in understanding the chemistry of cement hydration. Phil. Trans. R. Soc. London A310 (1983) 53–66.
P. Meredith, A. M. Donald and K. Luke. Pre-induction and induction hydration of tricalcium silicate: An environmental scanning electron microscope study. J. Mat. Sci. 30 (1995) 1921–1930.
H. N. Stein and J. M. Stevels. Influence of silica on the hydration of 3 CaO,SiO2. J. Appl. Chem. 14 (1964) 338–345.
J. G. de Jong and H. N. Stein, and J. M. Stevels. Hydration of tricalcium silicate. J. Appl. Chem. 17 (1967) 246–250.
T. C. Powers. Einige physikalische Gesichtspunkte zur Hydratation von Portlandzement. Zement-Kalk-Gips 14 (1961) 81–87.
D. D. Double, A. Hellawell and S. J. Perry. The hydration of Portland cement. Proc. R. Soc. London A369 (1978) 435–451.
D. D. Lasic, M. M. Pintar and R. Blinc. Are proton NMR observations supportive of the osmotic model of cement hydration?. Phil. Mag. Lett. 58 (1988) 227–232.
L. D. Mitchelle, M. Prica and J. D. Birchall. Aspects of Portland cement hydration studied using atomic force microscopy. J. Mat. Sci. 31 (1996) 4207–4212.
J. S. Lota, J. Bensted, J. Munn and P. L. Pratt. Hydration of class G oilwell cement at 20 ºC and 5 ºC. L'industria italiana del Cemento 725 (1997) 776–798.
D. Damidot and A. Nonat. Hydration of C3S. In: A. Nonat and J. C. Mutin, (eds), Hydration and Setting of Cements. London: E & F Spon (1992) 23–34.
D. Bentz, E. J. Garboczi, M. F. Kleyn and P. E. Stuzman. Cellular automaton simulations of cement hydration and microstructural development. Modelling Simul. Mater. Sci. Eng. 2 (1994) 783–808.
F. Tzschichholz, H. J. Herrmann and H. Zanni. Reaction-diffusion model for the hydration and setting of cement. Phys. Rev., E53 (1996) 2629–2637.
R. Kondo and S. Ueda. Kinetics and mechanisms of the hydration of cements. In Proc. Int. Conf. Chem. Cem.. Toyko (1968) 102–108.
J. M. Pommersheim and J. R. Clifton. Mathematical modelling of tricalcium silicate hydration. Cem. Conc. Res. 9 (1979) 765–770.
J. M. Pommersheim, J. R. Clifton. and G. J. Frohnsdorff. Mathematical modelling of tricalcium silicate hydration. II. Hydration sub-models and the effect of model parameters. Cem. Conc. Res. 12 (1982) 765–772.
P. Barret and, D. Bertrandie. Fundamental hydration kinetic features of the major cement constituents-Ca3SiO5 and β-Ca2SiO4. J. Chim. Phys. 82 (1986) 765–775.
S. A. Greenberg, T. N. Chang and E. Anderson. Investigation of colloidal hydrated calcium silicates. I. Solubility products. J. Phys. Chem. 64 (1960) 1151–1157.
N. L. Thomas and D. D. Double. Calcium and silicon concentrations in solution during the early stages of hydration of portland cement and tricalcium silicate. Cem. Conc. Res. 11 (1981) 675–687.
K. Fujii, and M. Kondo. Hydration of tricalcium silicate in a very early stage. In: Proc. Int. Conf. Chem. Cem.. Toyko (1968) 206–212.
R. A. Alberty and R. J. Silbey. Physical Chemistry. New York: Wiley and Sons (1992) 898 p.
H. F. W. Taylor. Cement Chemistry. London: Academic Press (1997) 499 p.
P. W. Brown, J. M. Pommersheim and G. J. Frohnsdorff. A kinetic model for the hydration of tricalcium silicate. Cem. Conc. Res 15 (1985) 35–41.
D. Damidot, A. Nonat and P. Barret. Kinetics of tricalcium silicate hydration in diluted suspensions by microcalorimetric measurements. J. Am. Ceram. Soc. 73 (1990) 3319–3322.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Preece, S., Billingham, J. & King, A. On the initial stages of cement hydration. Journal of Engineering Mathematics 40, 43–58 (2001). https://doi.org/10.1023/A:1017533810329
Issue Date:
DOI: https://doi.org/10.1023/A:1017533810329