Abstract
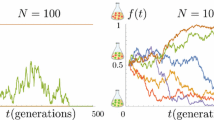
A model is developed for alternate fixations of mildly deleterious and wild-type alleles arising by forward and reverse mutation in a finite population. For almost all parameter values, this gives an equilibrium load that agrees closely with the general expression derived from diffusion theory. Nearly neutral mutations with selection coefficient a few times larger than 1/(2Ne) do the most damage by increasing the equilibrium load. The model of alternate fixations facilitates dynamical analysis of the expected load and the mean time to extinction in a population that has been suddenly reduced from a very large size to a small size. Reverse mutation can substantially improve population viability, increasing the mean time to extinction by an order of magnitude or more, but because many mutations are irreversible the effects may not be large. Populations with initially high mean fitness and small effective size, Ne below a few hundred individuals, may be at serious risk of extinction from fixation of deleterious mutations within 103 to 104 generations.
Similar content being viewed by others
References
Abramowitz, M. & I.A. Stegun, eds., 1972. Handbook of Mathematical Functions. Dover, New York.
Crow, J.F., 1993. Mutation, mean fitness, and genetic load. Oxford Surv. Evol. Biol. 9: 3-42.
Crow, J.F., 1970. An Introduction to Population Genetics Theory. Harper & Row, New York.
Crow, J.F.& M.J. Simmons, 1983. The mutation load in Drosophila, pp. 1-35 in The genetics and biology of Drosophila, edited byM. Ashburner, H.L. Carson & J.N. Thompson, Jr. Vol. 3c. Academic Press, New York.
Dobzhansky, Th., 1970. Genetics of the Evolutionary Process. Columbia University Press, New York.
Franklin, I.R., 1980. Evolutionary change in small populations, pp. 135-150. in Conservation biology, an evolutionaryecological perspective, edited by M.E. Soul´e & B.A. Wilcox. Sinauer, Sunderland, Mass.
Gregory, W.C., 1965. Mutation frequency, magnitude of change and the probability of improvement in adaptation. Radiation Botany 5 (Suppl.): 429-441.
Haldane, J.B.S., 1937. The effect of variation on fitness. Am. Nat. 71: 337-349.
Houle, D., D.K. Hoffmaster, S. Assimacopoulous & B. Charlesworth, 1992. The genomic mutation rate for fitness. Nature 359: 58-60.
Houle, D., K.A. Hughes, D.A. Hoffmaster, J. Ihara, S. Assimacopoulos, D. Canada & B. Charlesworth, 1994. The effects of spontaneousmutation on quantitative traits. I.Variances and covariances of life history traits. Genetics 138: 773-785.
Johnston, M.O. & D.J. Schoen, 1995. Mutation rates and dominance levels of genes affecting total fitness in two angiosperm species. Science 267: 226-229.
Keightley, P.D., 1994. The distribution of mutation effects on viability in Drosophila melanogaster. Genetics 138: 1315-1322.
Kimura, M., 1979. Model of effectively neutral mutations in which selective constraint is incorporated. Proc. Natl. Acad. Sci. USA 75: 1934-1937.
Kimura, M., T. Maruyama & J.F. Crow, 1963. The mutation load in small populations. Genetics 48: 1303-1312.
Kimura, M. & T. Ohta, 1969. The average number of generations until fixation of a mutant gene in a finite population. Genetics 61: 763-771.
Lande, R., 1988. Genetics and demography in biological conservation. Science 241: 1455-1460
Lande, R., 1994. Risk of population extinction from fixation of new deleterious mutations. Evolution 48: 1460-1469.
Lande, R., 1995. Mutation and conservation. Conserv. Biol. 9: 782-791.
Lynch, M., J. Conery & R. Bürger, 1995a. Mutation accumulation and the extinction of small populations. Am. Nat. 146: 489-518.
Lynch, M., J. Conery & R. Bürger, 1995b. Mutational meltdown in sexual populations. Evolution 49: 1067-1080.
Mackay, T.F.C., R.F. Lyman & M.S. Jackson, 1992. Effects of Pelement insertions on quantitative traits in Drosophila melanogaster. Genetics 130: 315-332.
Mukai, T., S.I. Chigusa, L.E. Mettler & J.F. Crow, 1972. Mutation rate and dominance of genes affecting viability in Drosophila melanogaster. Genetics 72: 335-355.
Mukai, T., 1979. Polygenic mutation, pp. 177-196 in Quantitative genetic variation, edited by J.N. Thompson, Jr. & J.M. Thoday. Academic Press, New York.
Muller, H.J., 1950. Our load of mutations. Am. J. Hum. Genet. 2: 111-176.
Muller, H.J. & I.I. Oster, 1956. Principles of back mutation as observed in Drosophila and other organisms, pp. 407-413 in Proc. Intl. Conf. Radiobiol., Stockholm.
Ohnishi, O., 1977a. Spontaneous and ethyl methanesulfonateinduced mutations controlling viability in Drosophila melanogaster. II. Homozygous effect of polygenic mutations. Genetics 87: 529-545.
Ohnishi, O., 1977b. Spontaneous and ethyl methanesulfonateinduced mutations controlling viability in Drosophila melanogaster. II. Heterozygous effect of polygenic mutations. Genetics 87: 547-556.
Santiago, E., J. Albornoz, A. Dominguez, M.A. Toro & C. LopezFanjul, 1992. The distribution of effects of spontaneous mutations on quantitative traits and fitness. Genetics 132: 771-781.
Schlager, G. & M.M. Dickie, 1971. Natural mutation rates in the house mouse. Estimates for five specific loci and dominant mutations. Mutat. Res. 11: 89-96.
Schultz, S.T. & M. Lynch, 1997. Mutation and extinction: the role of variable mutational effects, synergistic epistasis, beneficial mutations, and degree of outcrossing. Evolution 51: 1363-1371.
Shrimpton, A.E. & A. Robertson, 1988. The isolation of polygenic factors controlling bristle score in Drosophila melanogaster. Genetics 118: 445-459.
Simmons, M.J. & J.F. Crow, 1977. Mutations affecting fitness in Drosophila populations. Annu. Rev. Genet. 11: 49-78.
Soulé, M.E., 1980. Thresholds for survival: maintaining fitness and evolutionary potential, pp. 151-170 in Conservation biology, an evolutionaryecological perspective, edited by M.E. Soulé & B. A. Wilcox. Sinauer, Sunderland, Mass.
Yanovsky, C., H. Berger & W.J. Brammer, 1969. In vivo studies on the genetic code. Proc. XII. Intl. Congr. Genet. 3: 155-165.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lande, R. Risk of population extinction from fixation of deleterious and reverse mutations. Genetica 102, 21–27 (1998). https://doi.org/10.1023/A:1017018405648
Issue Date:
DOI: https://doi.org/10.1023/A:1017018405648