Abstract
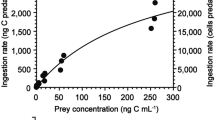
Laboratory experiments were performed to study the feeding behavior of Cyclops vicinus (Copepoda, Cyclopoida), fed two ciliates (Cyclidium sp. and Tetrahymena corlissi) chosen for their different size and swimming behavior. All grazing experiments were conducted with predators starved for 24 hr. Cyclidium sp. was fixed with Glutaraldehyde and Tetrahymena corlissi with mercuric chlorid in order to reduce counting errors. The Incipient Limit Level (ILL) for Tetrahymena corlissi was reached at the initial concentration of 76000 cell l-1 and corresponded to an ingestion rate of 340 cell ind-1 h-1; that for Cyclidium sp. was reached at 12 000 cell l-1 with an ingestion rate of 54 cell ind-1 h-1. The detection experiments were based on visual observations of Cyclops movements in experimental chambers with prey concentrations corresponding to the ILL. Both ciliates were used to evaluate the mechanisms involved in the detection of prey by Cyclops vicinus. Direct observations showed that Tetrahymena corlissi had a significant attractive effect on C. vicinus. Such behavior suggests that mechanoreception may be the overriding mechanism of remote detection. In contrast, C. vicinus does not react to the presence of Cyclidium sp. (at low concentration). These small prey, which swim actively in comparison with T. corlissi, are preyed on haphazardly. Ciliates can be a significant part of the diet of Cyclops vicinus, confirming their importance as mediators for energy transfer from the microbial loop to higher trophic levels; the mechanism of detection of such motile prey by Cyclops vicinus varies with prey swimming behavior.
Similar content being viewed by others
References
Adrian, R., 1987. Viability of phytoplankton in fecal pellets of two cyclopoid copepods. Arch. Hydrobiol. 110: 321–330.
Adrian, R., 1991 Filtering and feeding rates of cyclopoid copepods feeding on phytoplankton. Hydrobiologia 210: 217–223.
Archbold, H. G. & J. Berger, 1985. A qualitative assessment of some metazoan predators of Halteria grandinella a common freshwater ciliate. Hydrobiologia 124: 97–102.
Atema, J., 1987. Chemoreception in the sea: adaptations of chemoreceptors and behavior to aquatic stimulus conditions. In Atema, J., R. R. Ray, A. N. Popper & W. N. Tavolga (eds), Sensor Biology of Aquatic Animals. Springer, New York: 29–56.
Atkinson, A., 1996. Subantarctic copepods in an oceanic, low chlorophyll environment: ciliate predation, food selectivity and impact on prey population. Mar. Ecol. Prog. Ser. 130: 85–96.
Bark, A. W., 1985. Studies on ciliated protozoa in eutrophic lakes: 1. Seasonal distribution in relation to thermal stratification and hypolimnic anoxia. Hydrobiologia 124: 167–176.
Beaver, J. R. & T. L. Crisman, 1982. The trophic response of ciliated protozoans in freshwater lakes. Limnol. Oceanogr. 27: 246–253.
Beaver, J. R. & T. L. Crisman, 1989. The role of ciliated protozoa in pelagic freshwater ecosystems. Microb. Ecol. 17: 111–136.
Beaver, J. R., T. L. Crisman & J. R. W. Bienert, 1988. Distribution of planktonic ciliates in highly coloured subtropical lakes: comparison with clearwater ciliate communities and the contribution of mixotrophic taxa to total autotrophic biomass. Freshwat. Biol. 20: 51–60.
Bereczky, M. C., 1985. Fixationsund F¨arbungsschnellverfahren bei quantitativen ökologischen Untersuchungen von Protozoen in Binnengewässern. Arch. Protistenkd. 129: 187–190.
Berk, S. G., D. R. Brownlee, D. L. Heinle, H. J. King & R. R. Colwell, 1977. Ciliates as food source for marine planktonic copepods. Microbiol. Ecol. 4: 27–40.
Berninger, U. G., S. A. Wickham & B. J. Finlay, 1993. Trophic coupling within the microbial food web: a study with fine temporal resolution in a eutrophic freshwater ecosystem. Freshwat. Biol. 30: 419–432.
Bienert, R. W., J. R. Beaver & T. L. Crisman, 1991. The contribution of zooplankton biomass in an acidic, subtropical lake. J. Protozool. 38: 352–354.
Brandl, Z. & C.H. Fernando, 1975. Food consumption and utilization in two freshwater cyclopoid copepods (Mesocyclops edax and Cyclops vicinus). Int. Rev. Ges. Hydrobiol. 60: 471–494.
Cowles, T. J., R. J. Olson & S. W. Chishlom, 1988. Food selection by copepods: discrimination on the basis of food quality. Mar. Biol. 100: 41–49.
De Mott, W. R., 1988. Discrimination between algae and detritus by freshwater and marine zooplankton. Bull. Mar. Sci. 43: 486–499.
De Mott, W. R., 1989. Optimal foraging theory as predictor of chemically mediated food selection by suspension - feeding copepods. Limnol. Oceanogr. 34: 140–154.
De Mott, W. R., 1990. Retention efficiency, perceptual bias, and active choice as mechanism of food selection by suspensionfeeding zooplankton. In Hughes, N.N. (ed.), Behavioral Mechanism of Food Selection. NATO Series G: Ecological Sciences Springer, Heidelberg, New York: 569–594.
De Mott, W. R. & F. Moxter, 1991. Foraging on cyanobacteria by copepods: responses to chemical defenses and resource abundance. Ecology 72: 1820–1834.
De Mott, W. R. & M. D. Watson, 1991. Remote detection of algae by copepods: responses to algal size, odors and motility. J. Plankton Res. 13: 1203–1222.
Finlay, B. J., 1977. The dependence of reproductive rate on cell size and temperature in freshwater ciliated protozoa. Oecologia 30: 75–81.
Finlay, B. J., K. J. Clarke, A. J. Cowling, R. A. Hindle, A. Rogerson & U. G. Berninger, 1988. On the abundance and distribution of protozoa and their food in a productive freshwater pond. Eur. J. Protistol. 23: 205–217.
Folt, C., P. C. Schulze & K. Baumgartner, 1993. Characterising a zooplankton neighbourhood: small scale patterns of association and abundance. Freshwat. Biol. 30: 289–300.
Friedman, M. M., 1980. Comparative morphology and functional significance of copepod receptors and oral structures. In Kerfoot, W. C. (ed.), Evolution and Ecology of ZooplanktonCommunities. The University Press of New England, Hanover (N.H.); Lond.: 185–197.
Fulton, R. S. & H.W. Paerl, 1987. Toxic and inhibitory effects of the blue algae Microcystis aeruginosa on herbivorous zooplankton. J. Plankton Res. 9: 837–855.
Gates, M. A., 1984. Quantitative importance in the planktonic biomass of lake ecosystems. Hydrobiologia 108: 233–238.
Gifford, D. J., 1991. The protozoanmetazoan trophic link in pelagic ecosystems. J. Protozool. 38: 81–86.
Gilbert, J. J. & K. G. Bogdan, 1981. Selectivity of Polyarthra and Keratella for flagellate and aflagellate cells. Verh. int. Ver. Theor. Angew. Limnol. 21: 1515–1521.
Gliwicz, Z. M. & G. Umana, 1994. Cladoceran body size and vulnerability to copepod predation. Limnol. Oceanogr. 29: 419–424.
Hartmann, H. J., H. Taleb, L. Aleya & N. Lair, 1993. Predation on ciliates by the suspensionfeeding calanoid copepod Acanthodiaptomus denticornis. Can. J. Fish. aquat. Sci. 50: 1382–1393.
Hasset, R. P. & M. R. Landry, 1988. Shortterm changes in feeding and digestion by the copepod Calanus pacificus. Mar. Biol. 99: 63–74.
Holling, C. S., 1959. The components of predation as revealed by a study of smallmammal predation of the European pine sawfly. Can. J. Ent. 91: 293–320.
Hunt, G.H. & S. M. Chein, 1983. Seasonal distribution, composition and abundance of the planktonic ciliata and testacea of Cayuga lake. Hydrobiologia 98: 257–266.
Hutchinson, G. E., 1975. A treatise on limnology, 3. Wiley & Sons, New York, 660 pp.
Jonsson, P. R. & P. Tiselius, 1990. Feeding behavior, prey detection and capture efficiency of the copepod Acartia tonsa feeding on planktonic ciliates. Mar. Ecol. Prog. Ser. 60: 35–44.
Kerfoot, W. C., 1978. Combat between predatory copepods and their prey: Cyclops, Epischura, and Bosmina. Limnol. Oceanogr. 23: 1089–102.
Lair, N., 1992. Daytime grazing and assimilation rates of planktonic copepods Acanthodiaptomus denticornis and Cyclops vicinus vicinus.Comparison of spatial and resources utilisation by rotifers and cladocerans communities in an eutrophic lake. Hydrobiologia 231: 107–117.
Lloyd, M., 1967. 'Meancrowding'. J. Anim. Ecol. 36: 1–30.
Marin, V., M. E. Huntley & B. Frost, 1986. Measuring feeding rates of pelagic herbivores: analysis of experimental design and methods. Mar. Biol. 93: 49–58.
Pace, M. L., 1982. Planktonic ciliates: their distribution, abundance, and relationship to microbial resources in a monomictic lake. Can. J. Fish. aquat. Sci. 39: 1106–1116.
Pace, M. L., 1986. An empirical analysis of zooplankton community size structure across lake trophic gradients. Limnol.Oceanogr. 31: 45–55.
Pace M. L. & J. D. Orcutt, 1981. The relative importance of protozoans, rotifers and crustaceans in a freshwater zooplankton community. Limnol. Oceanogr. 26: 822–830.
Paffenhöfer, G. A. & K. B. Van Sant, 1986. The feeding response of a marine planktonic copepod to quantity and quality of particles. Mar. Ecol. Prog. Ser. 27: 55–65.
Price, H. J., 1988. Feeding mechanism in marine and freshwater zooplankton. Bull. mar. Sci. 43: 327–343.
Price, H. J., G. A. Paffenhöfer & J. R. Strickler, 1983. Modes of cell capture in calanoid copepods. Limnol. Oceanogr. 28: 116–123.
Runge, J. A., 1980. Effect of hunger and season on the feeding behavior of Calanus pacificus. Limnol. Oceanogr. 25: 134–145.
Sanders, R. W. & S. A. Wickham, 1993. Planktonic protozoa and metazoa: predation, food quality and population control. Mar. Microb. Food Webs 7: 197–223.
Santer, B., 1993. Potential importance of algae in the diet of adult Cyclops vicinus. Freshwat. Biol. 30: 269–278.
Santer, B., 1994. Influence of food type and concentration on the development of Eudiaptomus gracilis and implications for interactions between calanoid and cyclopoid copepods. Arch. Hydrobiol. 131: 141–159.
Santer, B. & F. van den Bosch, 1994. Herbivorous nutrition of Cyclops vicinus: the effect of a pure algal diet on feeding, development, reproduction and life cycle. J. Plankton Res. 16: 171–195.
Sherr, E. B., B. F. Sherr & G. A. Paffenhöfer, 1986. Phagotrophic protozoa as food for metazoans: a missing trophic link in marine pelagic food webs? Mar. Microb. Food Webs 1: 61–80.
Sherr, E. B., B. F. Sherr, T. Berman & O. Haddas, 1991. High abundance of picoplanktoningesting ciliates during late fall in lake Kinneret, Israel. J. Plankton Res. 13: 789–799.
SimeNgando, T. & H. J. Hartmann, 1991. Shortterm variations of the abundance and biomass of planktonic ciliates in an eutrophic lake. Eur. J. Protistol. 27: 249–263.
Stemberger, R. S., 1985. Prey selection by the copepod Diacyclops thomasi. Oecologia 65: 492–497.
Stœcker, D. K. & J. M. Capuzzo, 1990. Predation on protozoa: its importance to zooplankton. J. Plankton Res. 12: 891–908.
Stœcker, D. K. & D. A. Egloff, 1987. Predation by Acartia tonsa Dana on planktonic ciliates and rotifers. J. exp. mar. Biol. Ecol. 110: 53–68.
Strickler, J. R., 1985. Feeding currents in calanoid copepods: two new hypotheses. In Laverak, M. S. (ed.), Physiological Adaptations of Marine Animals. Soc. exp. Biol. 459–485.
Uchima, M., 1988. Gut content analysis of neritic copepods Acartia omorii and Oithona davisae by a new method. Mar. Ecol. Prog. Ser. 48: 93–97.
Uchima, M. & R. Hirano, 1986. Food ofOithona davisae (Copepoda: Cyclopoida) and the effect of food concentration at first feeding on the larval growth. Bull. Plankton Soc. Jap. 33: 21–28.
Vanderploeg, H. A. & G. A. Paffenhöfer, 1985. Modes of algal capture by the freshwater copepod Diaptomus sicilis and their relation to foodsize selection. Limnol. Oceanogr. 30: 871–885.
Vanderploeg, H. A., G. A. Paffenhöfer & J. R. Liebig, 1988. Diaptomus versus net phytoplankton: effects of algal size andmorphology on selectivity of a behaviorally flexible, omnivorous copepod. Bull. Mar. Sci. 43: 377–394.
Vijverberg, J., 1989. Culture techniques for studies on the growth, development and reproduction of copepods and cladocerans under laboratory and in situ conditions: a review. Freshwat. Biol. 21: 317–373.
Wickham, S. A, 1995. Cyclops predation on ciliates: speciesspecific differences and functional responses. J. Plankton Res. 17: 1633–1646.
Williamson, C. E., 1980. The predatory behavior of Mesocyclops edax: predator preferences, prey defenses, and starvationinduced changes. Limnol. Oceanogr. 25: 903–909.
Williamson, C. E., 1987. Predatorprey interactions between omnivorous diaptomid copepods and rotifers: the role of prey morphology and behavior. Limnol. Oceanogr. 32: 167–177.
Williamson, C. E., 1991. Copepoda: Ecology and classification of North American Freshwater Invertebrates 21: 787–822.
Williamson, C. E. & M. E. Stœckel, 1990. Estimating predation risk in zooplankton communities: the importance of vertical overlap. Hydrobiologia 198: 125–131.
Rights and permissions
About this article
Cite this article
Rabette, C., Thouvenot, A. & Lair, N. Laboratory experiments on trophic relationships and remote detection between two ciliates and Cyclops vicinus vicinus. Hydrobiologia 373, 157–167 (1998). https://doi.org/10.1023/A:1017001725062
Issue Date:
DOI: https://doi.org/10.1023/A:1017001725062