Abstract
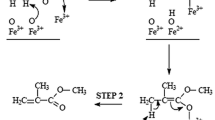
Isobutyric acid (IBA) oxidative dehydrogenation to methacrylic acid (MAA) reaction has been studied for a wide variety of iron (hydrogen) phosphates catalysts and mechanical mixtures of several of these phosphates. It was observed that the reaction needs high amounts of water, typically 10–12 mol water per mol IBA, to get stable, active and selective catalysts and follows a Mars and van Krevelen mechanism, as schematised below:
The best catalysts were found to be composed of trimers of face sharing FeO6octahedra, isolated one from the others by cationic vacancies and bounded to the next row by PO4tetrahedra. Solids having dimers or long chain octahedra are also active but less selective. Theoretical calculations showed that this corresponds to an electron hopping from one Fe2+to the next Fe3+cation during the redox mechanism for a trimer, while no electron hopping occurs for dimers and whereas it occurs all over the Fe cations for infinite octahedra chains. The presence of such vacancies and, to a lesser extent, of excess phosphorus at the surface, as shown by XPS analysis, exemplifies the site isolation concept in partial oxidation reactions. At variance, the cooperation effect between several phases was not observed.
Similar content being viewed by others
References
J.-M.M. Millet, Catal. Rev. Sci. Eng. 40 (1998) 1.
C. Marchal-Roch, N. Laronze, R. Villanneau, N. Guillou, A. Tézé and G. Hervé, J. Catal. 190 (2000) 173.
F. Ruszala, US patent 4 410 727 (1983), assigned to Ashland Oil Inc.
P. Bonnet, J.-M.M. Millet, C. Leclercq and J.C. Védrine, J. Catal. 158 (1996) 128.
J.-M.M. Millet and J.C. Védrine, Appl. Catal. 76 (1991) 209.
J.-M.M. Millet and B. Mentzen, Eur. J. Solid State Chem. 28 (1991) 493.
J.-M.M. Millet, M. Forissier, D. Rouzies, P. Bonnet and J.C. Védrine, Stud. Surf. Sci. Catal. 101 (1996) 1011.
V. Robert, S.A. Borshch and B. Bigot, J. Phys. Chem. 100 (1996) 580.
J.C. Védrine, J.-M.M. Millet and S.A. Borshch, Il Nuovo Cimento 19 (1997) 1759.
P.A. Aaskar, L. Decaul and R.K. Grasselli, Catal. Lett. 23 (1994) 339.
R.G. Teller, J.F. Brazdil, W. Yelon and R.K. Grasselli, J. Chem. Soc. 81 (1985) 1693.
R.K. Grasselli, Appl. Catal. 15 (1985) 127.
J.C. Védrine, J.-M. Millet and J.C. Volta, Faraday Discuss Chem. Soc. 87 (1989) 207.
J.C. Védrine, Stud. Surf. Sci. Catal. 110 (1997) 60.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Millet, JM.M., Védrine, J.C. Importance of site isolation in the oxidation of isobutyric acid to methacrylic acid on iron phosphate catalysts. Topics in Catalysis 15, 139–144 (2001). https://doi.org/10.1023/A:1016648516984
Issue Date:
DOI: https://doi.org/10.1023/A:1016648516984