Abstract
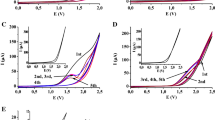
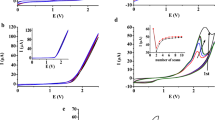
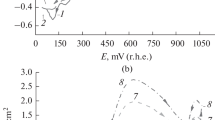
The electropolymerization of phenol and chlorinated phenols (monochlorophenols, dichlorophenols, 2,3,6-, 2,4,6-, 2,4,5-trichlorophenols and pentachlorophenol) was studied on a platinum electrode at 0.78 V vs SHE in alkaline 1 M NaOH aqueous solutions containing 0.1 M of the phenols. The low molecular weight reaction products were investigated by means of gas chromatography mass spectrometry (GCMS). Product analyses show that oligomers (dimers, trimers and tetramers) are present in the polymer mixtures formed. The MS spectra reveal the ether-linked nature of the oligomers formed during the electrooxidation-electropolymerization of the phenolic compounds. The mass spectra of the low molecular weight substances formed suggest that the oxidation–polymerization of phenols proceeds following two different mechanisms: (i) through the quinol-ether route (without chlorine elimination) and (ii) via the nucleophilic-radical substitution (SRN1) route (with some elimination of chlorine from ortho and/or para positions).
Similar content being viewed by others
References
G.D. Cooper, H.S. Blanchard, G.F. Endres and H. Finkbeiner, J. Am. Chem. Soc. 87 (1965) 3996.
G.D. Cooper and J.G. Bennet Jr, J. Org. Chem. 37 (1972) 441.
M. Karhu, J. Chem. Soc. Perkin Trans. 1 (1981) 303.
G. Mengoli, S. Daolio and M.M. Musiani, J. Appl. Electrochem. 10 (1980) 459.
M. Gattrell and B. MacDougall, J. Electrochem. Soc. 146 (1999) 3335.
P.I. Iotov and S.V. Kalcheva, J. Electroanal. Chem. 442 (1998) 19.
G. Mengoli and M. Musiani, J. Electrochem. Soc. 134 (1987) 643C.
M. Gattrell and D.W. Kirk, J. Electrochem. Soc. 140 (1993) 903.
M. Gattrell and D.W. Kirk, J. Electrochem. Soc. 139 (1992) 2736.
M. Gattrell and D.W. Kirk, J. Electrochem. Soc. 140 (1993) 1534.
J. Wang, M. Jiang and F. Lu, J. Electroanal. Chem. 444 (1998) 127.
F. Bruno, M.C. Phamand J.E. Dubois, Electrochim. Acta 22 (1977) 451.
S.H. Glarum, J.H. Marshall, M.Y. Hellman and G.N. Taylor, J. Electrochem. Soc. 134 (1987) 81.
S. Taj, M.F. Ahmed and S. Sankarapapavinasam, J. Electroanal. Chem. 356 (1993) 269.
R. Magnusson, Acta Chem. Scand. 18 (1964) 759.
E. McNelis, J. Am. Chem. Soc. 88 (1966) 1074.
G.D. Staffin and C.C. Price, J. Am. Chem. Soc. 82 (1960) 3632.
R.A. Rossi and R.H. Rossi, ‘Aromatic substitution by the SRN1 mechanism’ (Russian edition, Moscow, 1986).
I.P. Beleckaja and V.N. Drozd, Uspekhi Khimii 48 (1979) 793 (in Russian).
M.M. Baizer, ‘Organic Electrochemistry’ (Marcel Dekker, New York, 1973).
Z. Ežerskis and Z. Jusys, J. Appl. Electrochem. 31 (2001) 1117.
Z. Ežerskis, G. Stalnionis and Z. Jusys, J. Appl. Electrochem., 32 (2002) 49.
Z. Ežerskis and Z. Jusys, J. Appl. Electrochem., in press.
W. Vielstich, ‘Brennstoffelemente’ (VCH, Weinheim 1965).
H. Angerstein-Kozlowska, B.E. Conway and W.B.A. Sharp, J. Electroanal. Chem. 43 (1973) 9.
T.J. Stone, W.A. Waters, J. Chem. Soc. (1964) 213.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ežerskis, Z., Jusys, Z. Electropolymerization of chlorinated phenols on a Pt electrode in alkaline solution. Part IV: A gas chromatography mass spectrometry study. Journal of Applied Electrochemistry 32, 543–550 (2002). https://doi.org/10.1023/A:1016548627078
Issue Date:
DOI: https://doi.org/10.1023/A:1016548627078