Abstract
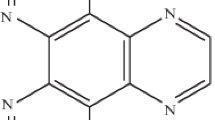
The characteristics of the in vitro interaction of cyanonaphthyridinomycin (CYANO) with DNA are described. Unlike naphthyridinomycin (NAP), CYANO is extremely dependent on reductive activation with dithiothreitol (DTT) to bind DNA. The reaction of CYANO with DNA is kinetically slower than that observed for NAP and is still linear after six hours incubation at room temperature. The extent of binding is pH dependent with acidic pH being inhibitory. CYANO, as with NAP, appears to bind to dG:dC base pairs in the minor groove of double stranded DNA. Studies using [C3H3:14CN] CYANO demonstrated that the cyanide group is lost when the drug binds to DNA. In the absence of DNA but in the presence of DTT, cyanide is still released from CYANO and the extent of release is also inhibited by acid pH conditions. These results suggest that the cyanide group comes off prior to binding of the antibiotic to DNA. The rate limiting step in the reaction of CYANO with DNA would appear to be the release of cyanide from the drug molecule.
Similar content being viewed by others
References
Kluepfel, D., Baker, H. A., Piattoni, G., Sehgal, S. N., Sidorowicz, A., Singh, K., Vezina, C. (1975) J. Antibiotics 28, 497–502.
Singh, K., Sun, S., Kleupfel, D. (1976) Develop. in Indus. Microb. 17, 209–221.
Zmijewski, M. J., Miller-Hatch, K., Goebel, M. (1982) Antimicrob. Ag. Chemo. 21, 787–793.
Zmijewski, Jr., M. J., Miller-Hatch, K., Mikolajczak, M. (1984) Accepted for publication, Chemico-Bio. Interactions.
Petrusek, R. L., Anderson, G. L., Garner, T. F., Fannin, W. L., Kaplan, D. T., Zimmer, S. G., Hurley, L. H. (1981) Biochem. 20, 1111–1119.
Lown, J. W., Joshua, A. V., Lee, L. S. (1982) Biochem. 21, 419–428.
Ishiguro, K., Takahashi, K., Yazawa, K., Sakiyama, S., Arai, T. (1981) J. Biol. Chem. 256, 2162–2167.
Zmijewski, Jr., M. J., Goebel, M. (1982) J. Antibiotics 35, 524–526.
Zmijewski, Jr., M. J., Mikolajczak, M. (1983) J. Antibiotics 36, 1767–1769.
Itoh, J., Omoto, S., Inouye, S., Kodama, Y., Hisamatsu, T., Niida, T., Ogawa, Y. (1982) J. Antibiotics 35, 642–644.
Hayashi, T., Noto, T., Nawata, Y., Ikazaki, H., Sawada, M., Ando, K. (1982) J. Antibiotics 35, 771–777.
Hayashi, T., Okutomi, T., Suzuki, S., Okazaki, H. (1983) J. Antibiotics 36, 1228–1235.
Zmijewski, Jr., M. J. Mikolajczak, M., Viswanatha, V., Hruby, V. J. (1982) J. Amer. Chem. Soc. 104, 4969–4971.
Subirana, J. A., Vives, J. L. (1981) Biopolymers 20, 2281.
Erickson, R. L., Szyblaski, W. (1984) Virology 22, 111–113.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zmijewski, M.J., Mikolajczak, M. The Interaction of Cyanonaphthyridinomycin with DNA. Pharm Res 2, 77–80 (1985). https://doi.org/10.1023/A:1016390728164
Issue Date:
DOI: https://doi.org/10.1023/A:1016390728164