Abstract
Purpose. A Monte Carlo simulation study was done to investigate the effects of high intrasubject variation in clearance (CL), and volume of distribution (V) on the calculation of the 90% confidence interval (CI) for Cmax for single dose and multiple dose studies.
Methods. Simulations were done for both immediate release and sustained release scenarios. The simulated data were compared with clinical data from bioequivalence studies performed on indomethacin and verapamil.
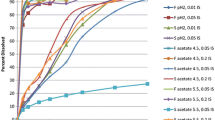
Results. Previous reviews and simulations have shown that the probability of failure for the Cmax for single dose studies was always greater than that for multiple dose studies. However, the results for the simulated scenarios currently investigated indicate that if intrasubject (period-to-period) variation in CL and V is high (% CV's above 25%, and 12%, respectively), multiple dose studies can exhibit a higher probability of failure for Cmax than do single dose studies. Furthermore, Cmax values from studies performed with a sustained release scenario are more sensitive to changes in Ka, CL, and V than are results of studies on immediate release products. As an example, the probability of failure for immediate release products in simulated single dose studies is about 11% and 21% when the mean difference in Ka is 10% and 20%, respectively; while, the probability of failure for multiple dose studies is about 36% regardless of the difference in Ka. The corresponding values for the probability of failure for sustained release products were 25%, 53% for single dose studies and 39% for multiple dose studies. The simulations also indicate that changes in the fraction absorbed have a greater effect on the estimation of Cmax in multiple dose regimens than in single dose studies.
Conclusions. The results from these investigations indicate that multiple dose studies do not necessarily always reduce variability in Cmax.
Similar content being viewed by others
REFERENCES
K. K. Midha and H. H. Blume, Bio-International, Medpharm, Stuttgart, German (1993).
Bio-International-94, Munich Germany, June 15–17, (1994).
AAPS regional workshop on highly variable drugs, Washington, D.C., (March 1995).
A. A. El-Tahtawy, A. J. Jackson, and T. M. Ludden. Comparison of single and multiple dose pharmacokinetics using clinical bioequivalence data and Monte Carlo simulations. Pharm. Res. 11, 1330 (1994).
M. Gibaldi and Perrier, In Pharmacokinetics, Marcel Dekker, New York, (1982).
D. J. Schuirmann. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability, J. of Pharmacokinetics and Biopharmaceutics. 15, 657 (1987).
H. H. Blume, B. Scheidel, and M. Siewert. Application of single-dose vs multiple-dose studies. K. K. Midha and H. H. Blume (eds.), In Bio-International, Medpharm, Stuttgart, Germany, pp 37–52. (1993).
F. Y. Bois, T. N. Tozer, W. W. Hauck, M. L. Chen, R. Patnaik, and R. L. Williams. Bioequivalence: performance of several measures of rate of absorption. J. Pharmaceutical Research. 11, 966 (1994).
L. B. Sheiner. Bioequivalence revisited. In Statistics in Medicine. 11, 1777 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
El-Tahtawy, A.A., Jackson, A.J. & Ludden, T.M. Evaluation of Bioequivalence of Highly Variable Drugs Using Monte Carlo Simulations. I. Estimation of Rate of Absorption for Single and Multiple Dose Trials Using Cmax. Pharm Res 12, 1634–1641 (1995). https://doi.org/10.1023/A:1016288916410
Issue Date:
DOI: https://doi.org/10.1023/A:1016288916410