Abstract
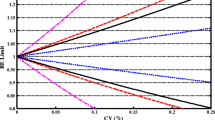
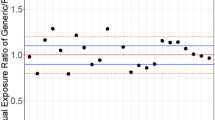
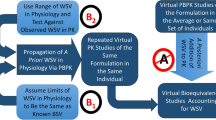
The aims of the proposed study were to develop and verify a quantitative model-based framework to anticipate the in vivo bioequivalence of ibuprofen immediate release formulations. This stepwise approach integrated virtual bioequivalence trials to simulate the test to reference (T/R) ratio for positive (i.e., bioequivalent) and negative (i.e., non-bioequivalent) control formulations containing ibuprofen, approximated distribution of interoccasion variability (IOV) on ibuprofen peak (Cmax) and extent of exposure (AUC) by bootstrapping resampling methods, post hoc incorporation of IOV to simulated T/R ratios, and power curve analysis. After post hoc incorporation of the bootstrapped IOV to the simulated Cmax T/R geometric mean ratios, the resulting 90% confidence intervals overlapped with the in vivo observations for both pairwise comparisons. On the other hand, simulated and observed AUC TNBE/R geometric mean ratios differed, likely due to the lack of propagating clearance-related IOV to the simulations. This approach is in line with modern regulatory initiatives that advocate leveraging quantitative methods and modeling to modernize generic drug development and review.

Graphical abstract




Similar content being viewed by others
References
Amidon GL, Lennernäs H, Shah VP, Crison JR. Theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62:1607–21.
Grimm M, Koziolek M, Kühn JP, Weitschies W. Interindividual and intraindividual variability of fasted state gastric fluid volume and gastric emptying of water. Eur J Pharm Biopharm. 2018;127:309–17.
U.S. Food & Drug Administration (FDA). Bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an ANDA — Guidance for Industry. 2013. https://www.fda.gov/files/drugs/published/Bioequivalence-Studies-With-Pharmacokinetic-Endpoints-for-Drugs-Submitted-Under-an-Abbreviated-New-Drug-Application.pdf. Accessed 03 March 2020.
U.S. Food & Drug Administration (FDA). Guidance for Industry SUPAC-MR: modified release solid oral dosage forms scale-up and postapproval changes: Chemistry, Manufacturing, and Controls; In Vitro Dissolution Testing and In Vivo Bioequivalence Documentation. 1997. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/supac-mr-modified-release-solid-oral-dosage-forms-scale-and-postapproval-changes-chemistry. Accessed 03 March 2020.
European Medicines Agency (EMA). Guideline On The Investigation of Bioequivalence. 2010. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf. Accessed 03 March 2020.
U.S. Food & Drug Administration (FDA). Statistical approaches to establishing bioequivalence. 2001. https://www.fda.gov/media/70958/download. Accessed 19 June 2020.
Moreno I, Ochoa D, Román M, Cabaleiro T, Abad-Santos F. Utility of pilot studies for predicting ratios and intrasubject variability in high-variability drugs. Basic Clin Pharmacol Toxicol. 2016;119:215–21.
Heimbach T, Suarez-Sharp S, Kakhi M, Holmstock N, Olivares-Morales A, Pepin X, et al. Dissolution and translational modeling strategies toward establishing an in vitro-in vivo link-a workshop summary report. AAPS J. 2019;21(2):29.
Fang L, Kim MJ, Li Z, Wang Y, DiLiberti CE, Au J, et al. Model-informed drug development and review for generic products: summary of FDA public workshop. Clin Pharmacol Ther. 2018;104:27–30.
Butler J, Hens B, Vertzoni M, Brouwers J, Berben P, Dressman J, et al. In vitro models for the prediction of in vivo performance of oral dosage forms: recent progress from partnership through the IMI OrBiTo collaboration. Eur J Pharm Biopharm. 2019;136:70–83.
Loisios-Konstantinidis I, Cristofoletti R, Fotaki N, Turner DB, Dressman J. Establishing virtual bioequivalence and clinically relevant specifications using in vitro biorelevant dissolution testing and physiologically-based population pharmacokinetic modeling. case example: Naproxen. Eur J Pharm Sci. 2020. https://doi.org/10.1016/j.ejps.2019.105170.
Sumner T, et al. Methodology for global-sensitivity analysis of time-dependent outputs in systems biology modelling. J R Soc Interface. 2012;9:2156–66.
Cristofoletti R, Dressman JB. Bridging the gap between in vitro dissolution and the time course of ibuprofen-mediating pain relief. J Pharm Sci. 2016;105(12):3658–67.
Morris MD. Factorial sampling plans for preliminary computational experiments. Technimetrics. 1999;33(2):161–74.
Cristofoletti R, Dressman JB. FaSSIF-V3, but not compendial media, appropriately detects differences in the peak and extent of exposure between reference and test formulations of ibuprofen. Eur J Pharm Biopharm. 2016;105:134–40.
Fuchs A, Leigh M, Kloefer B, Dressman JB. Advances in the design of fasted state simulating intestinal fluids: FaSSIF-V3. Eur J Pharm Biopharm. 2015;94:229–40.
Pathak SM, Ruff A, Kostewicz ES, Patel N, Turner DB, Jamei M. Model-based analysis of biopharmaceutic experiments to improve mechanistic oral absorption modeling: an integrated in vitro in vivo extrapolation perspective using ketoconazole as a model drug. Mol Pharm. 2017;14:4305–20.
Cristofoletti R, Hens B, Patel N, Esteban VV, Schmidt S, Dressman J. Integrating drug- and formulation-related properties with gastrointestinal tract variability using a product-specific particle size approach: case example ibuprofen. J Pharm Sci. 2019;108:3842–7.
Le VNP, et al. Influence of granulation and compaction on the particle size of ibuprofen--development of a size analysis method. Int J Pharm. 2006;321:72–7.
Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A. The Simcyp population-based ADME simulator. Expert Opin Drug Metab Toxicol. 2009;5:211–23.
Kolewe ME, Roberts SC, Henson MA. A population balance equation model of aggregation dynamics in Taxus suspension cell cultures. Biotechnol Bioeng. 2012;109:472–82.
Martin W, Koselowske G, Töberich H, Kerkmann T, Mangold B, Augustin J. Pharmacokinetics and absolute bioavailability of ibuprofen after oral administration of ibuprofen lysine in man. Biopharm Drug Dispos. 1990;11(3):265–78.
Abduljalil K, Cain T, Humphries H, Rostami-Hodjegan A. Deciding on success criteria for predictability of pharmacokinetic parameters from in vitro studies: an analysis based on in vivo observations. Drug Metab Dispos. 2014;42(9):1478–84.
Guest EJ, Aarons L, Houston JB, Rostami-Hodjegan A, Galetin A. Critique of the two-fold measure of prediction success for ratios: application for the assessment of drug-drug interactions. Drug Metab Dispos. 2011;39(2):170–3.
R Core Team. R: A language and environment for statistical computing. 2019. https://www.R-project.org/. Accessed 03 March 2020.
Legg TJ, Laurent AL, Leyva R, Kellstein D. Ibuprofen sodium is absorbed faster than standard ibuprofen tablets: results of two open-label, randomized, crossover pharmacokinetic studies. Drugs R D. 2014;14(4):283–90.
Troconiz IF, Armenteros S, Planelles MV, Benitez J, Calvos R, Dominguez R. Pharmacokinetic-pharmacodynamic modelling of the antipyretic effect of two oral formulations of ibuprofen. Clin Pharmacokinet. 2001;38(6):505–18.
Sugano K, Terada K. Rate- and extent-limiting factors of oral drug absorption: theory and applications. J Pharm Sci. 2015;104(9):2777–88.
Blume H, Mutschler E. Bioaquivalenz, Qualitatsbewertung wirkstoffgleicher Fertigarzneimittel, Teil I/II, Isosorbiddinitrat 6. Erg¨anzungslieferung, Govi-Verlag Pharmazeutischer Verlag, Frankfurt/Main-Eschborn; 1996.
Lozoya-Agullo I, Araújo F, González-Álvarez I, Merino-Sanjuán M, González-Álvarez M, Bermejo M, et al. PLGA nanoparticles are effective to control the colonic release and absorption on ibuprofen. Eur J Pharm Sci. 2018;115:119–25.
Atkinson HC, Stanescu I, Frampton C, Salem II, Beasley CPH, Robson R. Pharmacokinetics and bioavailability of a fixed-dose combination of ibuprofen and paracetamol after intravenous and Oral administration. Clin Drug Investig. 2015;35:625–32.
Davit BM, Nwakama PE, Buehler GJ, Conner DP, Haidar SH, Patel DT, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583–97.
Mitra A. Maximizing the role of physiologically based Oral absorption modeling in generic drug development. Clin Pharmacol Ther. 2019;105:307–9.
Lionberger RA. Innovation for generic drugs: science and research under the generic drug user fee amendments of 2012. Clin Pharmacol Ther. 2019;105:878–85.
Lionberger RA. Decision science for generic drug development and review. J Clin Pharmacol. 2019;59:1249–51.
Manolis E, Musuamba FT, Karlsson KE. Regulatory considerations for building an in silico clinical pharmacology backbone by 2030. Clin Pharmacol Ther. 2020;107:746–8. https://doi.org/10.1002/cpt.1772.
Acknowledgments
The authors thank Simcyp® Limited for providing an academic license of the Simcyp® Simulator v18.2 and 19.1 as well as the license of the SIVA® toolkit v3 to the Center for Pharmacometrics and Systems Pharmacology, University of Florida without charge. Bart Hens acknowledges the Flemish Research Council (FWO: applicant number 12R2119N). Ioannis Loisios-Konstantinidis would like to thank the European Union’s Horizon 2020 Research and Innovation Program under grant agreement No 674909 (PEARRL).
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
I.L.K., B.H, A.M., S.K., C.C., and R.C. wrote the manuscript; R.C. designed the research; I.L.K., B.H. and R.C. performed the research; I.L.K., B.H., S.K., C.C., and R.C. analyzed the data.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ioannis Loisios-Konstantinidis and Bart Hens are equal first authors
Rights and permissions
About this article
Cite this article
Loisios-Konstantinidis, I., Hens, B., Mitra, A. et al. Using Physiologically Based Pharmacokinetic Modeling to Assess the Risks of Failing Bioequivalence Criteria: a Tale of Two Ibuprofen Products. AAPS J 22, 113 (2020). https://doi.org/10.1208/s12248-020-00495-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00495-4