Abstract
Purpose. The in vitro fate of an ester prodrug, glycovir, was studied to determine if the species differences in the bioavailability of pharmacologically active SC-48334 observed after glycovir administration and not observed after SC-48334 administration is due to species differences in ester hydrolysis rate or species differences in absorption of the prodrug itself, and to determine the site(s) of ester hydrolysis which contributes most to species differences in the bioavailability of SC-48334 if any.
Methods. Glycovir was incubated with small intestinal mucosa, liver S9 fractions, whole blood, red blood cells (RBC) and plasma of the rat, dog, monkey (cynomolgus and rhesus) and man, and glycovir concentrations were determined by HPLC.
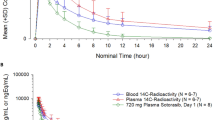
Results. The relative bioavailabilities of SC-48334 after prodrug administration to the rat, dog, monkey and man were 99,15, 42 and 37%, respectively. After SC-48334 administration, SC-48334 was rapidly and similarly well absorbed in all species. The hydrolysis rate in the small intestinal mucosa was well correlated with the relative bioavailability of SC-48334 after prodrug administration. Among different species the hydrolysis rate of glycovir in liver S9 fractions, blood, RBC and plasma did not parallel those in the mucosa of the small intestine.
Conclusions. The species differences in bioavailability of SC-48334 with the prodrug were due to species differences in hydrolysis rates of the prodrug in small intestinal mucosa. The monkey was a good animal model for prediction of esterase activity in human small intestine and relative bioavailability in man.
Similar content being viewed by others
REFERENCES
A. Karpas, G.W.J. Fleet, R.A. Dwek, S. Petursson, S.K. Namgoong, N.G. Ramsden, G.S. Jacob and T.W. Rademacker. Aminosugar Derivatives as Potential Anti-Human Immunodeficiency Virus Agents. Proc. Ntl. Acad. Sci. 85:9229–9233 (1988).
M. Narawane, S.K. Podder, H. Bundgaard, and V.H.L. Lee. Segmental Differences in Drug Permeability, Esterase Activity and Ketone Reductase Activity in the Albino Rabbit Intestine. J. Drug Target. 1:29–39 (1993).
M. Miyauch, T. Hirota, K. Fujimoto, and J. Ide, Studies on Orally Active Cephalosporin Esters. IV. Effect of the C-3 Substituent of Cephalosporin on the Gastrointestinal Absorption in Mice. Chem. Pharm. Bull. 37:3272–3276 (1989).
A.A. Sinhura, and S.H. Yalkowsky. Rationale for design of biologically reversible drug derivatives; Prodrugs. J. Pharm. Sci. 64:181–210 (1975).
M. Rowland, S. Riegelman, P.A. Harris and S.D. Sholkoff. Absorption of aspirin kinetics in man following oral administration of an aqueous solution. J. Pharm. Sci. 61:379–385 (1972).
M. Inoue, M. Morikawa, M. Tsuboi and M. Sugiura. Species difference and characterization of intestinal esterase on the hydrolyzing activity of ester-type drugs. Jpn. J. Pharmacol. 29:9–16 (1979).
W.E. Luttrell and M.C. Castle. Species difference in the hydrolysis of meperidine and its inhibition by organophosphate compounds. Fundam. Appl. Toxicol. 11:323–332 (1988).
M. Morikawa, M. Inoue and M. Tsuboi. Substrate specificity of carboxylesterase from several animals. Chem. Pharm. Bull. 24:1661–1664 (1976).
C.Y. Quon, K. Mai, G. Patil, and H.F. Stampfli. Species differences in the stereoselective hydrolysis of esmol by blood esterases. Drug Metab. Dispos. 16:425–428 (1988).
S.K. Yang, Y. Hsieh, W. Chang and J. Huang. Enantioselectivity of microsomal and cytosolic esterases in rat intestinal mucosa. Drug Metab. Dispos. 20:719–725 (1992).
C.S. Cook, C. Hauswald, J.A. Oppermann and G.L. Schoenhard. Involvement of Cytochrome P-450IIIA in Metabolism of Potassium Canrenoate to an Epoxide: Mechanism of inhibition of the epoxide formation by spironolactone and its sulfur-containing metabolite. J. Pharmacol. Exp. Ther. 266:1–7 (1993).
W.N. Aldridge. Serum esterase. I. Two types of esterases (A and B) hydrolyzing p-nitrophenyl acetate, propionate and butyrate, and a method for determination. Biochem. J. 53:110–117 (1953).
W.N. Aldridge. A-esterase and B-esterase in perspective. In E. Reiner, W.N. Aldridge and E.C.G. Hoskin (eds.), Enzymes Hydrolyzing Organophosphorus Compounds, Ellis Horwood, Chichester, 1989, pp. 246–253.
A.H. Conney. Pharmacological implication of microsomal enzyme induction. Pharmacol. Rev. 19:317–366 (1967).
F.M. Williams. Clinical significance of esterases in man. Clin. Pharmacokinet. 10:392–403 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cook, C.S., Karabatsos, P.J., Schoenhard, G.L. et al. Species Dependent Esterase Activities for Hydrolysis of an Anti-HIV Prodrug Glycovir and Bioavailability of Active SC-48334. Pharm Res 12, 1158–1164 (1995). https://doi.org/10.1023/A:1016259826037
Issue Date:
DOI: https://doi.org/10.1023/A:1016259826037