Abstract
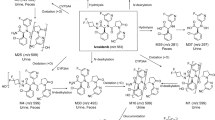
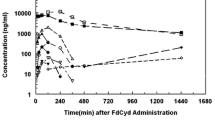
IDX184 is a phosphoramidate prodrug of 2′-methylguanosine-5′-monophosphate, developed to treat patients infected with hepatitis C virus. A mass balance study of radiolabeled IDX184 and pharmacokinetic studies of IDX184 in portal vein-cannulated monkeys revealed relatively low IDX184 absorption but higher exposure of IDX184 in the portal vein than in the systemic circulation, indicating >90 % of the absorbed dose was subject to hepatic extraction. Systemic exposures to the main metabolite, 2′-methylguanosine (2′-MeG), were used as a surrogate for liver levels of the pharmacologically active entity 2′-MeG triphosphate, and accordingly, systemic levels of 2′-MeG in the monkey were used to optimize formulations for further clinical development of IDX184. Capsule formulations of IDX184 delivered acceptable levels of 2′-MeG in humans; however, the encapsulation process introduced low levels of the genotoxic impurity ethylene sulfide (ES), which necessitated formulation optimization. Animal pharmacokinetic data guided the development of a tablet with trace levels of ES and pharmacokinetic performance equal to that of the clinical capsule in the monkey. Under fed conditions in humans, the new tablet formulation showed similar exposure to the capsule used in prior clinical trials.






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the plasma concentration-time curve
- BQL:
-
Below the limit of quantification
- C max :
-
Maximum observed plasma concentration
- CI:
-
Confidence interval
- ES:
-
Ethylene sulfide
- FaSSGF/FeSSGF:
-
Fasted or fed-state simulated gastric fluid
- FaSSIF/FeSSIF:
-
Fasted or fed-state simulated intestinal fluid
- HCV:
-
Hepatitis C virus
- 2′-MeG:
-
2′-Methylguanosine
- MP:
-
Monophosphate
- NI:
-
Nucleoside inhibitors
- PEG:
-
Polyethylene glycol
- ppm:
-
Parts per million
- T 1/2 :
-
Apparent terminal half-life
- TP:
-
Triphosphate
- TTC:
-
Threshold of toxicological concern
References
Cretton-Scott E, Perigaud C, Peyrottes S, Licklider L, Camire M, Larsson M, et al. In vitro antiviral activity and pharmacology of IDX184, a novel and potent inhibitor of HCV replication. J Hepatol. 2008;48(Suppl 2):S220.
European Medicines Agency (EMA). Guideline on the limits of genotoxic impurities. 2006.
European Medicines Agency (EMA). Questions and answers on the ‘Guideline on the limits of genotoxic impurities’. 2010.
Freeman DJ, Grant DR, Carruthers SG. The cyclosporin–erythromycin interaction: impaired first pass metabolism in the pig. Br J Pharmacol. 1991;103:1709–12.
Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for Hepatitis C. N Engl J Med. 2013;368:34–44.
Hagan LM, Schinazi RF. Best strategies for global HCV eradication. Liver Int. 2013;33:68–79.
INCIVEK® (telaprevir). Prescribing information. 2013. http://pi.vrtx.com/files/uspi_telaprevir.pdf. Accessed 19 Mar 2015.
Lalezari J, Asmuth D, Casiró A, Vargas H, Lawrence S, Dubuc-Patrick G, et al. Short-term monotherapy with IDX184, a liver-targeted nucleotide polymerase Inhibitor, in patients with Chronic Hepatitis C Virus infection. Antimicrob Agents Chemother. 2012;56(12):6372–8.
Le Pogam S, Yan JM, Chhabra M, Ilnicka M, Kang H, Kosaka A, et al. Characterization of Hepatitis C Virus (HCV) Quasispecies dynamics upon short-term dual therapy with the HCV NS5B nucleoside polymerase inhibitor mericitabine and the NS3/4 protease inhibitor danoprevir. Antimicrob Agents Chemother. 2012;56:5494–502.
Migliaccio G, Tomassini JE, Carroll SS, Tomei L, Altamura S, Bhat B, et al. Characterization of resistance to non-obligate chain-terminating ribonucleoside analogs that inhibit Hepatitis C Virus replication in vitro. J Biol Chem. 2003;278(49):49164–70.
Murakami E, Tolstykh T, Bao H, Niu C, Steuer HMM, Bao D, et al. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J Biol Chem. 2010;285(45):34337–47.
OLYSIO™ (simeprevir). Prescribing information. 2014. http://www.olysio.com/shared/product/olysio/prescribing-information.pdf. Accessed 19 Mar 2015.
SOVALDI™ (sofosbuvir). Prescribing information. 2014. http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/sovaldi/sovaldi_pi.pdf. Accessed 19 Mar 2015
Standring D. IDX184 and novel nucleotides for the treatment of HCV. 20th International round table on nucleosides, nucleotides and nucleic acids, Montreal, Canada, August 8th, 2012.
U.S. Food and Drug Administration (FDA). Guidance for industry genotoxic and carcinogenic impurities in drug substances and products: recommended approaches. 2008.
U.S. Food and Drug Administration (FDA). M7 Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk. 2013.
Vernachio JH, Bleiman B, Bryant KD, Chamberlain S, Hunley D, Hutchins J, et al. INX-08189, a phosphoramidate prodrug of 6-O-methyl-2′-C-methyl guanosine, is a potent inhibitor of Hepatitis C Virus replication with excellent pharmacokinetic and pharmacodynamic properties. Antimicrob Agents Chemother. 2011;55(5):1843–51.
VICTRELIS® (boceprevir). Prescribing information. 2014. http://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf. Accessed 19 Mar 2015.
Zhou XJ, Pietropaolo K, Chen J, Khan S, Sullivan-Bólyai J, Mayers D. Safety and pharmacokinetics of IDX184, a liver-targeted nucleotide polymerase inhibitor of Hepatitis C Virus, in healthy subjects. Antimicrob Agents Chemother. 2011;55:76–81.
Acknowledgments
The authors would like to thank Emerson Resources for performing tablet development and GMP manufacturing, SNBL, Xenometrics and Covance for in-life phases of the in vivo studies, and PharmaNet USA for bioanalytical analyses. Also the authors would like to thank Dr. Xiao-Jian Zhou and other members of the Idenix clinical team for providing the clinical data as well as thank the healthy volunteers who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan-Zhou, XR., Mayes, B.A., Rashidzadeh, H. et al. Pharmacokinetics of IDX184, a liver-targeted oral prodrug of 2′-methylguanosine-5′-monophosphate, in the monkey and formulation optimization for human exposure. Eur J Drug Metab Pharmacokinet 41, 567–574 (2016). https://doi.org/10.1007/s13318-015-0267-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-015-0267-4