Abstract
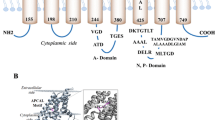
Plasmid pl258 carries the cadA gene that confers resistance to cadmium, lead, and zinc. CadA catalyzes ATP-dependent cadmium efflux from cells of Staphylococcus aureus. It is a member of the superfamily of P-type ATPases and belongs to the subfamily of soft metal ion pumps. In this study the membrane topology of this P-type ATPase was determined by constructing fusions with the topological reporter genes phoA or lacZ. A series of 44 C-terminal truncated CadAs were fused with one or the other reporter gene, and the activity of each chimeric protein was determined. In addition, the location of the first transmembrane segment was determined by immunoblot analysis. The results are consistent with the pl258 CadA ATPase having eight transmembrane segments. The first 109 residues is a cytosolic domain that includes the Cys(X)2Cys motif that distinguishes soft metal ion-translocating P-type ATPases from their hard metal ion-translocating homologues. Another feature of soft metal ion P-type ATPases is the CysProCys motif, which is found in the sixth transmembrane segment of CadA. The phosphorylation site and ATP binding domain conserved in all P-type ATPases are situated within the large cytoplasmic loop between the sixth and seventh transmembrane segments.
Similar content being viewed by others
REFERENCES
Axelsen, K. B., and Palmgren, M. G. (1998). J. Mol. Evol. 46, 84–101.
Bayle, D., Wangler, S., Weitzenegger, T., Steinhilber, W., Volz, J., Przybylski, M., Schafer, K. P., Sachs, G., and Melchers, K. (1998). J. Bacteriol. 180, 317–329.
Beard, S. J., Hashim, R., Membrillo-Hernandez, J., Hughes, M. N., and Poole, R. K. (1997). Mol. Microbiol. 25, 883–891.
Broome-Smith, J. K., Tadayyon, M., and Zhang, Y. (1990). Mol. Microbiol. 4, 1637–1644.
Chopra, I. (1975). Antimicrob. Agents Chemother. 7, 8–14.
DiDonato, M., Narindrasorasak, S., Forbes, J. R., Cox, D.W., and Sarkar, B. (1997). J. Biol. Chem. 272, 33279–33282.
Ehrmann, M., Bolek, P., Mondigler, M., Boyd, D., and Lange, R. (1997). Proc. Natl. Acad. Sci. USA 94, 13111–13115.
Ellis, J., Carlin, A., Steffes, C., Wu, J., Liu, J., and Rosen, B. P. (1995). Microbiology 141, 1927–1935.
Franke, C. M., Tiemersma, J., Venema, G., and Kok, J. (1999). J. Biol. Chem. 274, 8484–8490.
Froshauer, S., and Beckwith, J. (1984). J. Biol. Chem. 259, 10896–10903.
Gatti, D., Mitra, B., and Rosen, B. P. (2000). J. Biol. Chem. 275, 34009–34012.
Gershoni, J. M., and Palade, G. E. (1983). Anal. Biochem. 131, 1–15.
Guan, L., Ehrmann, M., Yoneyama, H., and Nakae, T. (1999). J. Biol. Chem. 274, 10517–10522.
Klein, P., Kanehisa, M., and DeLisi, C. (1985). Biochim. Biophys. Acta 815, 468–476.
Kyte, J., and Doolittle, R. F. (1982). J. Mol. Biol. 157, 105–132.
Laemmli, U. K. (1970). Nature 227, 680–685.
Lippard, S. J., and Berg, J. M. (1994). Principles of Bioinorganic Chemistry, University Science Books, Mill Valley, CA.
Lutsenko, S., and Kaplan, J. H. (1995). Biochemistry 34, 15607–15613.
Lutsenko, S., Petrukhin, K., Gilliam, T. C., and Kaplan, J. H. (1997a). Ann. NY Acad. Sci. 834, 155–157.
Lutsenko, S., Petrukhin, K., Cooper, M. J., Gilliam, C. T., and Kaplan, J. H. (1997b). J. Biol. Chem. 272, 18939–18944.
Manoil, C., and Beckwith, J. (1986). Science 233, 1403–1408.
Melchers, K., Herrmann, L., Mauch, F., Bayle, D., Heuermann, D., Weitzenegger, T., Schuhmacher, A., Sachs, G., Haas, R., Bode, G., Bensch, K., and Schafer, K. P. (1998). Acta Physiol. Scand. Suppl. 643, 123–135.
Melchers, K., Schuhmacher, A., Buhmann, A., Weitzenegger, T., Belin, D., Grau, S., and Ehrmann, M. (1999). Res. Microbiol. 150, 507–520.
Melchers, K., Weitzenegger, T., Buhmann, A., Steinhilber, W., Sachs, G., and Schafer,K. P. (1996). J. Biol. Chem. 271, 446–457.
Michaelis, S., Guarente, L., and Beckwith, J. (1983). J. Bacteriol. 154, 356–365.
Miller, J. (1992). A Short Course in Bacterial Genetic, Cold Spring Harbor Laboratory, New York.
Novick, R. P., and Roth, C. (1968). J. Bacteriol. 95, 1335–1342.
Nucifora, G., Chu, L., Misra, T. K., and Silver, S. (1989). Proc. Natl. Acad. Sci. USA 86, 3544–3548.
Rensing, C., Fan, B., Sharma, R., Mitra,B., and Rosen, B. P. (2000). Proc. Natl. Acad. Sci. USA 97, 652–656.
Rensing, C., Mitra, B., and Rosen, B. P. (1997). Proc. Natl. Acad. Sci. USA 94, 14326–14331.
Rensing, C., Sun, Y., Mitra, B., and Rosen, B. P. (1998). J. Biol. Chem. 273, 32614–32617.
Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989). Molecular Cloning, a Laboratory Manual, Cold Spring Harbor Laboratory, New York.
Silhavy, T. J., and Beckwith, J. R. (1985). Microbiol. Rev. 49, 398–418.
Silver, S., and Walderhaug, M. (1992). Microbiol. Rev. 56, 195–228.
Smith, D. L., Tao, T., and Maguire, M. E. (1993). J. Biol. Chem. 268, 22469–22479.
Solioz, M., and Odermatt, A. (1995). J. Biol. Chem. 270, 9217–9221.
Solioz, M., and Vulpe, C. (1996). Trends. Biochem. Sci. 21, 237–241.
Toyoshima, C., Nakasako, M., Nomura, H., and Ogawa, H. (2000). Nature 405, 647–655.
Tsai, K. J., Yoon, K. P., and Lynn, A. R. (1992). J. Bacteriol. 174, 116–121.
von Heijne, G. (1989). Nature 341, 456–458.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, KJ., Lin, YF., Wong, M.D. et al. Membrane Topology of the pl258 CadA Cd(II)/Pb(II)/Zn(II)-Translocating P-Type ATPase. J Bioenerg Biomembr 34, 147–156 (2002). https://doi.org/10.1023/A:1016085301323
Issue Date:
DOI: https://doi.org/10.1023/A:1016085301323