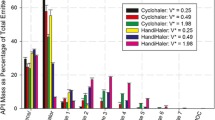
Abstract
Pressurized metered dose inhaler (MDI) output from three different albuterol formulations was characterized using three inertial separation devices. Results were compared for the Delron six-stage cascade impactor (DCI6), the Andersen Mark II eight-stage impactor (ACI8), and Copley's twin-stage liquid impinger (LI). None of the devices tested in this study was ideal in all respects. All devices could differentiate between formulations in terms of respirable doses (albuterol amount with aerodynamic diameters <5.5 through 6.4 µm). Only the high-flow rate LI could differentiate among all three formulations when data were presented in terms of respirable percentage (RP) of drug collected. Values for RP were in excellent agreement for the independently calibrated impactors when the same evaporation chamber was used atop the impactors. The LI appeared to overestimate values for RP in vivo. Results are discussed in light of the debate surrounding the revision of USP aerosol testing requirements. Rigorous specifications for evaporation chambers and methodologies are necessary for meaningful inter- and intra-laboratory comparison of results when any of these devices are used.
Similar content being viewed by others
REFERENCES
G. W. Hallworth and D. G. Westmoreland. The twin impinger: A simple device for assessing the delivery of drugs from metered dose pressurized aerosol inhalers. J. Pharm. Pharmacol. 39:966–972 (1987).
G. W. Hallworth and U. G. Andrew. Size analylsis of suspension inhalation aerosols by inertial separation methods. J. Pharm. Pharmacol. 28: 898–907 (1976).
G. W. Hallworth and R. R. Hamilton. Size analysis of metered suspension pressurized aerosols with the Quantimet 720. J. Pharm. Pharmacol. 28:890–897 (1976).
R. N. Dalby and P. R. Byron, Comparison of output particle size distributions form pressurized aerosols formulated as solutions or suspensions. Pharm. Res. 5:36–39 (1988).
P. R. Byron, R. N. Dalby, and A. J. Hickey. Optimized inhalation aerosols. I. The effects of spherical baffle size and position upon the output of several pressurized nonaqueous suspension formulations. Pharm. Res. 6:225–229 (1989).
D. Hathaway. Particle size measurement of an aerosol deodorant using laser holographic microscopy. Aerosol Age Sept. 28 (1973).
K. K. L. Ho, I. W. Kellaway, and R. L. Tredree. The determination of aerosol output during size analysis by laser diffraction. J. Pharm. Pharmacol. 39 (suppl.):77p (1987).
C. S. Kim, D. Trujillo, and M. A. Sackner. Size aspects of metered-dose inhaler aerosols. Am. Rev. Resp. Dis. 132:137–142 (1985).
K. R. May. J. Sci Instr. 22:187–195 (1945).
I. Porush. Pharmaceutical products. In H. R. Shepherd (ed.), Aerosols: Science and Technology, Interscience, New York, 1961, pp. 387–408.
FDA Division of Bioequivalence. Guidance for the in Vitro Portion of Bioequivalence Requirements for Metaproterenol Sulfate and Albuterol Inhalation Aerosols.
British Pharmacopoeia, p. 875, Appendix XVII C, A204-A207 (1988).
P. A. Haywood, M. Martin-Smith, J. M. Padfield, I. J. Smith, and R. N. Woodhouse. Establishing more meaningful specifications and tests for metered-dose pressurized inhalers formulated as suspensions. Pharm. Forum. 15(3):5193–5202 (1989).
USP XXII, Physical tests and determinations, p. 1556 (1990).
I. Gonda, J. B. Kayes, and F. J. T. Fildes. Characterization of hygroscopic inhalation aerosols. In N. G. Stanley-Wood and T. Allen (eds.), Particle Size Analysis, Wiley-Heyden, London, 1981, pp. 31 et seq.
Operating Manual for Andersen IACFM Non-Viable Ambient Particle Sizing Samplers, Fourth Revision, Andersen Samplers, Atlanta, GA, November 1985.
P. R. Byron. The prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J. Pharm. Sci. 75:433–438 (1986).
S. P. Newman. Deposition and dose determination from pressurized metered dose inhalers. Respiratory Drug Delivery II, Colorado, April 1990.
USP XXII, Metaproterenol sulfate inhalation aerosol, p. 836 (1990).
N. C. Miller. The effects of water in inhalation suspension aerosol formulations. In P. R. Byron (ed.), Respiratory Drug Delivery, CRC Press, Boca Raton, FL, 1990, pp. 249–257.
G. W. Snedecor and W. C. Cochran. Statistical Methods, 7th ed., Iowa State University Press, Ames, 1980.
J. P. Mitchell, P. A. Costa, and S. Waters. An assessment of an Andersen Mark II cascade impactor. J. Aerosol Sci. 19:213–221 (1988).
N. P. Vaughan. The Andersen impactor: Calibration, wall losses and numerical simulation. J. Aerosol Sci. 20:67–90 (1989).
B. M. Z. Zainudin, S. E. J. Tolfree, M. Biddiscombe, M. Whitaker, M. D. Short, and S. G. Spiro. An alternative to direct labelling of pressurised bronchodilator aerosol. Int. J. Pharm. 51:67–71 (1989).
G. P. Martin, A. E. Bell, and C. Marriott. An in vitro method for assessing particle deposition from metered pressurized aerosols and dry powder inhalers. Int. J. Pharm. 44:57(1988).
P. R. Byron. Aerosol formulation, generation and delivery using metered systems. In P. R. Byron (ed.), Respiratory Drug Delivery, CRC Press, Boca Raton, FL, 1990, p. 197.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Phillips, E.M., Byron, P.R., Fults, K. et al. Optimized Inhalation Aerosols. II. Inertial Testing Methods for Particle Size Analysis of Pressurized Inhalers. Pharm Res 7, 1228–1233 (1990). https://doi.org/10.1023/A:1015973402316
Issue Date:
DOI: https://doi.org/10.1023/A:1015973402316