Abstract
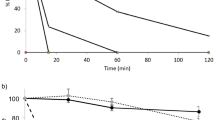
The stability of the neuroleptic peptide des-enkephalin-γ-endorphin (DEγE; Org 5878) in the rectal lumen and the rectal bioavailability of DEγE were investigated in conscious rats. Furthermore, the influence of peptidase inhibition, peptidase saturation, and absorption enhancement on DEγE bio-availability were evaluated. Na2EDTA (0.25%, w/v) prolonged the degradation half-life of DEγE in the ligated colon from 33 ± 7 to 93 ± 45 min. Without adjuvant, tritium-labeled DEγE was absorbed from the rat rectum to a very low extent (0–4%). After administration of an excess of unlabeled DEγE or with Na2EDTA, comparable results were obtained. The medium-chain glyceride preparation MGK markedly enhanced the rectal DEγE bioavailability, up to 8–20%, which was further increased to 10–44% by coadministration of Na2EDTA. No substantial influence of varying the rectal delivery rate was observed. The results suggest that absorption enhancement and enzyme inhibition both are essential for effective increase of rectal peptide bioavailability.
Similar content being viewed by others
REFERENCES
J. W. van Nispen and H. M. Greven. Pharm. Ther. 16:67–102 (1982).
D. de Wied. In E. R. de Kloet, V. M. Wiegant, and D. de Wied (eds.), Neuropeptides and Brain Function, Progr. Brain Res., Vol. 72, Elsevier, Amsterdam, 1987, pp. 93–108.
S. A. Adibi and Y. S. Kim. In L. R. Johnson (ed.), Physiology of the Gastrointestinal Tract, Raven Press, New York, 1981, pp. 1073–1095.
V. H. L. Lee. Pharm. Int. 7:208–212 (1986).
P. Gruber, M. A. Longer, and J. R. Robinson. Adv. Drug Del. Rev. 1:1–18 (1987).
M. J. Humphrey. In S. S. Davis, L. Illum, and E. Tomlinson (eds.), Delivery Systems for Peptide Drugs, Plenum Press, New York, 1986, pp. 139–151.
A. G. de Boer, F. Moolenaar, L. G. J. de Leede, and D. D. Breimer. Clin. Pharmacokin. 7:285–311 (1982).
L. G. J. de Leede, A. G. de Boer, C. P. J. M. Roozen, and D. D. Breimer. J. Pharmacol. Exp. Ther. 225:181–185 (1983).
A. K. Mitra. Pharm. Int. 323–324 (1986).
H. M. Jennewein, F. Waldeck, and W. Konz. Drug Res. 24:1225–1228 (1974).
M. Hirata, S. Futaguchi, T. Tamura, K. Odaguchi, and A. Tanaka. Chem. Pharm. Bull. 26:1061–1065 (1978).
M. Saito, T. Kumasaki, Y. Yaoi, N. Nishi, A. Arimura, D. H. Coy, and A. V. Schally. Fertil. Steril. 28:240–245 (1977).
T. Mitsuma and T. Nogimori. Acta Endocrinol. 107:207–212 (1984).
W. A. Ritschel and G. B. Ritschel. Meth. Find. Exp. Clin. Pharmacol. 6:513–529 (1984).
V. Bocci, F. Corradeschi, A. Naldini, and E. Lencioni. Int. J. Pharm. 34:111–114 (1986).
I. Yamazaki. In F. Labrie, A. Belanger, and A. Dupont (eds.), LHRH and Its Analogues, Elsevier, Amsterdam, 1984, pp. 77–91.
J. A. Moore, S. A. Pletcher, and M. J. Ross. Int. J. Pharm. 34:35–43 (1986).
K. Morimoto, H. Akatsuchi, R. Aikawa, M. Morishita, and K. Morisaka. J. Pharm. Sci. 73:1366–1368 (1984).
T. Yagi, N. Hakui, Y. Yamasaki, R. Kawamori, M. Shichiri, H. Abe, S. Kim, M. Miyake, K. Kamikawa, T. Nishihata, and A. Kamada. J. Pharm. Pharmacol. 35:177–178 (1983).
M. Sekine, K. Sasahara, R. Okada, and S. Awazu. J. Pharmacobio-Dyn. 8:645–652 (1985).
Y. P. Loh. Am. Rev. Neurosci. 7:189–222 (1984).
M. Sekine, K. Sasahara, T. Kojima, K. Hasegawa, R. Okada, and S. Awazu. J. Pharmacobio-Dyn. 7:856–863 (1984).
J. Nakamura, K. Shima, T. Kimura, S. Muranishi, and H. Sezaki. Chem. Pharm. Bull. 26:857–863 (1978).
E. J. van Hoogdalem, H. J. M. van Kan, A. G. de Boer, and D. D. Breimer. J. Controlled Release 7:53–60 (1988).
J. C. Verhoef and H. M. van den Wildenberg. Regul. Peptides 14:113–124 (1986).
J. C. Verhoef, H. M. van den Wildenberg, and J. W. van Nispen. Eur. J. Drug Metab. Pharmacokin. 11:291–302 (1986).
W. L. Chiou. J. Pharmacokin. Biopharm. 6:539–546 (1978).
E. J. van Hoogdalem, A. M. Stijnen, A. G. de Boer, and D. D. Breimer. J. Pharm. Pharmacol. 40:329–332 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Hoogdalem, E.J., Heijligers-Feijen, C.D., de Boer, A.G. et al. Rectal Absorption Enhancement of Des-Enkephalin-γ-Endorphin (DEγE) by Medium-Chain Glycerides and EDTA in Conscious Rats. Pharm Res 6, 91–95 (1989). https://doi.org/10.1023/A:1015864022305
Issue Date:
DOI: https://doi.org/10.1023/A:1015864022305