Abstract
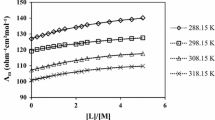
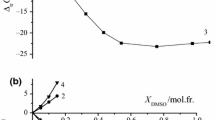
Rate constants and derived thermodynamic activation parameters are reported for solvolysis of trans-[Co(3Mepy)4Cl2]+ and [Co(CN)5Cl]3− ions in water-rich mixtures of water with ethanol at various temperatures and are analyzed by initial- and transition-state contributions. The variation of enthalpies and entropies of activation with solvent composition show extrema in composition ranges where the physical properties of the mixtures, influenced by changes in solvent structures, also show extrema. From the application of a free-energy cycle to the process of the initial state going to the transition state, it is concluded for the solvolysis of both complexes that the Co(III) species in the transition state is more stable in water + ethanol mixtures than in the initial state.
Similar content being viewed by others
REFERENCES
N. Nishi, S. Takahashi, M. Matsumoto, A. Tanaka, K. Muraya, T. Taramuku, and T. Yamaguchi, J. Phys. Chem. 99, 462(1995).
J. Burgess, J. Chem. Soc. A, p. 1899(1969).
M. J. Blandamer, J. Burgess, and R. I. Hains, J. Chem. Soc. Dalton. Trans., p. 612(1980).
G. M. El-Subruiti, Transition Met. Chem. 25, 219(2000).
G. M. El-Subruiti and S. S. Massoud, Transition Met. Chem. 25, 344(2000).
G. M. El-Subruiti, Transition Met. Chem. 22, 33(1997).
G. M. El-Subruiti, C. F. Wells, and I. M. Sidahmed, Intern. J. Chem. Kinetics 23, 161(1991).
C. F. Wells, Progr. Reaction Kinetics 20, 1(1995).
J. Burgess and E. Pelizzetti, Progr. Reaction Kinetics 17, 1(1992).
G. M. El-Subruiti, C. F. Wells, and I. M. Sidahmed, J. Solution Chem. 21, 93(1992).
S. Brownstein, Can. J. Chem. 38, 1590(1960).
J. A. Dilts and D. F. Shriver, J. Amer. Chem. Soc. 90, 5769(1968).
G. Akerlöf, J. Amer. Chem. Soc. 54, 4125(1932).
C. F. Wells, J. Chem. Soc. Faraday Trans. I 73, 1851(1977).
A. H. Fainberg and S. Winstein, J. Amer. Chem. Soc. 78, 2770(1956).
Z. Kebede and N. Retta, Transition Met. Chem. 15, 417(1990).
J. Burgess, J. Chem. Soc. A, p. 1085(1968) and references therein.
R. G. Wilkins, The Study of Kinetics and Mechanism of Reactions of Transition Metal Complexes (Allyn &; Bacon, Boston, MA, 1974), p. 97.
A. N. Acharya and A. C. Dash, J. Chem. Soc. Faraday Trans. 90, 3293(1994).
N. Nishi, K. Koga, C. Ohshima, K. Yamamato, U. Nagashima, and K. Nagami, J. Amer. Chem. Soc. 110, 5246(1988).
M. J. Blandamer, D. F. Clarke, N. J. Hidden, and M.C. Symons, Chem. Commun. p. 342(1966).
M. J. Blandamer, D. E. Clarke, T. A. Claxton, and M. F. Fox, Chem. Commun. p. 273(1967).
M. J. Blandamer, Advan. Phys. Org. Chem. 14, 203(1972).
J. Hao and A. J. Poë, Transition Met. Chem. 23, 739(1998).
C. F. Wells. J. Chem. Soc. Faraday Trans. I 80, 2445(1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
El-Subruiti, G.M. Kinetics and Thermodynamics of Solvolysis of a Cationic and an Anionic Chlorocobalt (III) Complex in Water–Ethanol Mixtures. Journal of Solution Chemistry 31, 415–423 (2002). https://doi.org/10.1023/A:1015863416229
Issue Date:
DOI: https://doi.org/10.1023/A:1015863416229