Abstract
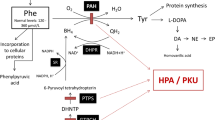
Phenylketonuria (PKU) is biochemically characterized by the accumulation of phenylalanine (Phe) and its metabolites in tissues of affected children. Neurological damage is the clinical hallmark of PKU, and Phe is considered the main neurotoxic metabolite in this disorder. However, the mechanisms of neurotoxicity are poorly known. The main objective of the present work was to measure the activities of the mitochondrial respiratory chain complexes (RCC) and succinate dehydrogenase (SDH) in brain cortex of Wistar rats subjected to chemically induced hyperphenylalaninemia (HPA). We also investigated the in vitro effect of Phe on SDH and RCC activities in the cerebral cortex of 22-day-old rats. HPA was induced by subcutaneous administration of 2.4 μmol/g body weight α-methylphenylalanine, a phenylalanine hydroxylase inhibitor, once a day, plus 5.2 μM/g body weight phenylalanine, twice a day, from the 6th-21st postnatal day. The results showed a reduction of SDH and complex I + III activity in brain cortex of rats subjected to HPA. We also verified that Phe inhibited the in vitro activity of complexes I + III, possibly by competition with NADH. Considering the importance of SDH and RCC for the maintenance of energy supply to brain, our results suggest that energy deficit may contribute to the Phe neurotoxicity in PKU.
Similar content being viewed by others
REFERENCES
Scriver, C. R. and Kaufman, S. 2001. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. Pages 1667–1724 in Scriver, C. R., Beaudet, A. L., Sly, W. S., Valle, D. (eds): The Metabolic & Molecular Bases of Inherited Diseases, 8th ed., McGraw-Hill, New York.
Rodrigues, N. R., Wannmacher, C. M. D., Dutra-Filho, C. S., Pires, R. F., Fagan, P. R., and Wajner, M. 1990. Effect of phenylalanine, p-chlorophenylalanine and a-methylphenylalanine on glucose uptake in vitro by the brain of young rats. Biochem. Soc. Trans. 18:419.
Wyse, A. T. S., Bolognesi, G., Brusque, A. M., Wajner, M., and Wannmacher, C. M. D. 1995. Na+, K+ -ATPase activity in the synaptic plasma membrane from the cerebral cortex of rats subjected to chemically induced phenylketonuria. Med. Sci. Res. 23:261–262.
De Freitas, M. S., de Mattos, A. G., Camargo, M. M., Wannmacher, C. M. D., and Pessoa-Pureu, R.. 1995. Effect of phenylalanine and α-methylphenylalanine on in vitro incorporation of 32P into cytoskeletal cerebral proteins. Neurochem. Int. 26:381–385.
Hasselbach, S., Knudsen, G. M., Toft, P. B., Hogh, P., Tedeschi, E., Holm, S., Videbaek, C., Henriksen, O., Lou, H.C., Paulson, O. B. 1996. Cerebral glucose metabolism is decreased in white matter changes in patients with phenylketonuria. Pediatr. Res. 40:21–24.
Bauman, M. L. and Kemper, T. L. 1982. Morphologic and histoanatomic observations of the brain in untreated human phenylketonuria. Acta. Neuropathol. 58:55–60.
Di Donato, S. 2000. Disorders related to mitochondrial membranes: pathology of the respiratory chain and neurodegeneration. J. Inher. Metab. Dis. 23:247–263.
Wallace, D. C. 1999. Mitochondrial diseases in man and mouse. Science 283:1482–1488.
Babcock, G. T., Wikström, M. 1992. Oxygen activation and the conservation of energy in cell respiration. Nature 356:301–309.
Wyse, A. T. S., Sarkis, J. J. F. S., Cunha-Filho, J. S., Teixeira, M. V., Schetinger, M. R., Wajner, M., and Wannmacher, C. M. D. 1994. Effect of phenylalanine and its metabolites on ATP diphosphohydrolase activity in synaptosomes from rat cerebral cortex. Neurochem. Res. 19:1175–1180.
Fischer, J. C., Ruitenbeek, W., Berden, J. A., Trijbels, J. M., Veerkamp, J. H., Stadhouders, A. M., Sengers, R. C., and Janssen, A. J., 1985. Differencial investigation of the capacity of succinate oxidation in human skeletal muscle. Clin. Chim. Acta 153:23–36.
Rustin, P., Chretien, D., Bourgeron, T., Gérard, B., Rötig, A., Saudubray, J. M., and Munnich, A. 1994. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta 228:35–51.
Schapira, A. H., Cooper, J. M., Dexter, D., Clark, J. B., Jenner, P., and Marsden, C. D. 1990. Mitochondrial complex I deficiency in Parkinson's disease. J. Neurochem. 54:823–827.
Sorensen, R. G. and Mahler, H. R. 1982. Localization of endogenous ATPases at the nerve terminal. J. Bioenerg. Biomembr. 14:527–547.
. Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Lê-Quôc, K., Lê-Quôc, D., and Gaudemer, Y. 1981. Evidence for the existence of two classes of sulfhydryl groups essential for membrane-bound succinate dehydrogenase activity. Biochemistry 20:1705–1710.
Kienzle-Hagen, M. E., Pederzolli, C. D., Sgaravatti, A. M., Bridi, R., Wajner, M., Wannmacher, C. M. D., Wyse, A. T. S., and Dutra-Filho, C. S. 2001. Experimental phenylketonuria provokes oxidative stress in rat brain. J. Inher. Metab. Dis. 24:131.
Sierra, C., Vilaseca, M. A., Moyano, D., Brandi, N., Campistol, J., Lambruschini, N. F. J., Cambra, F. J., Deulofeu, R., and Mira, A. 1998. Antioxidant status in hyperphenylalaninemia. Clin. Chim. Acta 276:1–9.
Weber, G. 1969. Inhibition of human brain pyruvate kinase and hexokinase by phenylalanine and phenylpiruvate: possible relevance to phenylketonuric brain damage. Proc. Natl. Acad. Sci. USA 63:1365–1369.
Tanji, K. and Bonilla, E. 2000. Neuropathological aspects of cytochrome c oxidase deficiency. Brain Pathol. 10:420–430.
Schapira, A. H. 1999. Mitochondrial involvement in Parkinson's disease, Huntington's disease, hereditary spastic paraplegia and Friedreich's ataxia. Biochim. Biophys. Acta 1410:159–170.
Ramsey, R. R., Krueger, M. J., Youngster, S. K., Gluck, M. R., Casida, J. E., and Singer, J. 1991. Interaction of 1–methyl-4–phenylpiridinium ion (MPP+) and its analogs with the rotenone/piericidin binding site of NADH dehydrogenase. J. Neurochem. 56:1184–1190.
Rutter, G. A. and Rizzuto, R. 2000. Regulation of mitochondrial metabolism by ERCa2+ release: an intimate connection. Trends Biochem. Sci. 25:215–221.
Di Lisa, F. and Bernardi, P. 1998. Mitochondrial function: a determinant of recovery or death in cell response to injury. Mol. Cell Biochem. 184:379–391.
Takeshiga, K. and Minakami, S. 1979. NADH and NADPHdependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem. J. 180:129–135.
Beyer, R. 1991. An analysis of the role of coenzyme Q in free radical generation and as an anti-oxidant. Biochem. Cell Biol. 70:343–390.
Robinson, B. H. 1994. Human complex I deficiency: clinical spectrum and involvement of oxygen free radicals in the pathogenicity of the defect. Biochim. Biophys. Acta 1364:271–276.
Pitkãnen, S. and Robinson, B. H. 1996. Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J. Clin. Invest. 98:345–351.
Chinopoulos, C. and Adam-Vizi, V. 2001. Mitochondria deficient in complex I activity are depolarized by hydrogen peroxide in nerve terminals: relevance to Parkinson's disease. J. Neurochem. 76:302–306.
Hasegawa, E., Takeshige, K., Oishi, T., Murai, Y., and Minikami, S. 1990. 1–Methyl-4–phenylpyridinium (MPP+) induces NADH-dependent superoxide formation and enhances NADH-dependent lipid peroxidation in bovine heart submitochondrial particles. Biochem. Biophys. Res. Commun. 170:1049–1055.
Beal, M. E. 2000. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 23:298–304.
Schinder, A. F., Olson, E. C., Spitzer, N. C., and Montal, M. 1996. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J. Neurosci. 16:6125–6133.
Massieu, L., Del Rio, P., and Montiel, T. 2001. Neurotoxicity of glutamate uptake inhibition in vivo: correlation with succinate dehydrogenase activity and prevention by energy substrates. Neuroscience 106:669–677.
Ferger, B., Eberhardt, O., Teismann, P., de Groote, C., and Schulz, J. B. 1999. Malonate-induced generation of reactive oxygen species in rat striatum depends on dopamine release but not on NMDA receptor activation. J. Neurochem. 17:1329–1332.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rech, V.C., Feksa, L.R., Dutra-Filho, C.S. et al. Inhibition of the Mitochondrial Respiratory Chain by Phenylalanine in Rat Cerebral Cortex. Neurochem Res 27, 353–357 (2002). https://doi.org/10.1023/A:1015529511664
Issue Date:
DOI: https://doi.org/10.1023/A:1015529511664