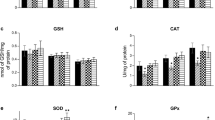
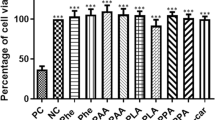
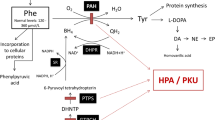
Abstract
Phenylketonuria (PKU) is a metabolic disorder accumulating phenylalanine (Phe) and its metabolites in plasma and tissues of the patients. Regardless of the mechanisms, which Phe causes brain impairment, are poorly understood, energy deficit may have linked to the neurotoxicity in PKU. It is widely recognized that creatine is involved in maintaining of cerebral energy homeostasis. Because of this, in a previous work, we incorporated it into liposomes and this increased the concentration of creatine in the cerebral cortex. Here, we examined the effect of creatine nanoliposomes on parameters of oxidative stress, enzymes of phosphoryl transfer network, and activities of the mitochondrial respiratory chain complexes (RCC) in the cerebral cortex of young rats chemically induced hyperphenylalaninemia (HPA). HPA was induced with l-phenylalanine (5.2 µmol/g body weight; twice a day; s.c.), and phenylalanine hydroxylase inhibitor, α-methylphenylalanine (2.4 µmol/g body weight; once a day; i.p.), from the 7th to the 19th day of life. HPA reduced the activities of pyruvate kinase, creatine kinase, and complex II + III of RCC in the cerebral cortex. Creatine nanoliposomes prevented the inhibition of the activities of the complexes II + III, caused by HPA, and changes oxidative profile in the cerebral cortex. Considering the importance of the mitochondrial respiratory chain for brain energy production, our results suggesting that these nanoparticles protect against neurotoxicity caused by HPA, and can be viable candidates for treating patients HPA.
Graphic abstract




Similar content being viewed by others
References
Adhihetty PJ, Beal MF (2008) Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. Neuromol Med 10(4):275–290. https://doi.org/10.1007/s12017-008-8053-y
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Allen PJ (2012) Creatine metabolism and psychiatric disorders: does creatine supplementation have therapeutic value? Neurosci Biobehav Rev 36(5):1442–1462. https://doi.org/10.1016/j.neubiorev.2012.03.005
Alshaer W, Hillaireau H, Vergnaud J, Ismail S, Fattal E (2015) Functionalizing liposomes with anti-CD44 Aptamer for selective targeting of cancer cells. Bioconjug Chem 26(7):1307–1313. https://doi.org/10.1021/bc5004313
Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR (2008) Functions and effects of creatine in the central nervous system. Brain Res Bull 76(4):329–343. https://doi.org/10.1016/j.brainresbull.2008.02.035
Balbino TA, Serafin JM, Radaic A, de Jesus MB, de la Torre LG (2017) Integrated microfluidic devices for the synthesis of nanoscale liposomes and lipoplexes. Colloids Surf B 152:406–413. https://doi.org/10.1016/j.colsurfb.2017.01.030
Berti SL, Nasi GM, Garcia C, Castro FL, Nunes ML, Rojas DB, Moraes TB, Dutra-Filho CS, Wannmacher CM (2012) Pyruvate and creatine prevent oxidative stress and behavioral alterations caused by phenylalanine administration into hippocampus of rats. Metab Brain Dis 27(1):79–89. https://doi.org/10.1007/s11011-011-9271-9
Bilder DA, Burton BK, Coon H, Leviton L, Ashworth J, Lundy BD, Vespa H, Bakian AV, Longo N (2013) Psychiatric symptoms in adults with phenylketonuria. Mol Genet Metab 108(3):155–160. https://doi.org/10.1016/j.ymgme.2012.12.006
Borin DB, Mezzomo NJ, Vaucher RA, Carmo GD, Rodrigues Junior LC, Sulczewski FB, Schwertz CI, Mendes RE, Damiani AP, Andrade VM, Rech VC, Boeck CR (2018) Production, characterization and toxicology assay of creatine pegylated nanoliposome with polysorbate 80 for brain delivery. An Acad Bras Cienc 90(2 suppl 1):2317–2329. https://doi.org/10.1590/0001-3765201820170553
Bortoluzzi VT, de Franceschi ID, Rieger E, Wannmacher CM (2014) Co-administration of creatine plus pyruvate prevents the effects of phenylalanine administration to female rats during pregnancy and lactation on enzymes activity of energy metabolism in cerebral cortex and hippocampus of the offspring. Neurochem Res 39(8):1594–1602. https://doi.org/10.1007/s11064-014-1353-8
Brewer GJ, Wallimann TW (2000) Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem 74(5):1968–1978
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352. https://doi.org/10.1385/0-89603-472-0:347
Brusque AM, Borba Rosa R, Schuck PF, Dalcin KB, Ribeiro CA, Silva CG, Wannmacher CM, Dutra-Filho CS, Wyse AT, Briones P, Wajner M (2002) Inhibition of the mitochondrial respiratory chain complex activities in rat cerebral cortex by methylmalonic acid. Neurochem Int 40(7):593–601
Busanello EN, Zanatta A, Tonin AM, Viegas CM, Vargas CR, Leipnitz G, Ribeiro CA, Wajner M (2013) Marked inhibition of Na + , K(+)- ATPase activity and the respiratory chain by phytanic acid in cerebellum from young rats: possible underlying mechanisms of cerebellar ataxia in Refsum disease. J Bioenerg Biomembr 45(1–2):137–144. https://doi.org/10.1007/s10863-012-9491-7
Christ SE, Price MH, Bodner KE, Saville C, Moffitt AJ, Peck D (2016) Morphometric analysis of gray matter integrity in individuals with early-treated phenylketonuria. Mol Genet Metab 118(1):3–8. https://doi.org/10.1016/j.ymgme.2016.02.004
Costabeber E, Kessler A, Severo Dutra-Filho C, de Souza Wyse AT, Wajner M, Wannmacher CM (2003) Hyperphenylalaninemia reduces creatine kinase activity in the cerebral cortex of rats. Int J Dev Neurosci 21(2):111–116
da Silva CG, Ribeiro CA, Leipnitz G, Dutra-Filho CS, Wyse AA, Wannmacher CM, Sarkis JJ, Jakobs C, Wajner M (2002) Inhibition of cytochrome c oxidase activity in rat cerebral cortex and human skeletal muscle by D-2-hydroxyglutaric acid in vitro. Biochim Biophys Acta 1586(1):81–91
Davies KJ (1995) Oxidative stress: the paradox of aerobic life. Biochem Soc Symp 61:1–31
de Franceschi ID, Rieger E, Vargas AP, Rojas DB, Campos AG, Rech VC, Feksa LR, Wannmacher CM (2013) Effect of leucine administration to female rats during pregnancy and lactation on oxidative stress and enzymes activities of phosphoryltransfer network in cerebral cortex and hippocampus of the offspring. Neurochem Res 38(3):632–643. https://doi.org/10.1007/s11064-012-0961-4
De Rosa G, De Stefano D, Laguardia V, Arpicco S, Simeon V, Carnuccio R, Fattal E (2008) Novel cationic liposome formulation for the delivery of an oligonucleotide decoy to NF-kappaB into activated macrophages. Eur J Pharm Biopharm 70(1):7–18. https://doi.org/10.1016/j.ejpb.2008.03.012
Di Donato S (2000) Disorders related to mitochondrial membranes: pathology of the respiratory chain and neurodegeneration. J Inherit Metab Dis 23(3):247–263
Diaz F, Garcia S, Padgett KR, Moraes CT (2012) A defect in the mitochondrial complex III, but not complex IV, triggers early ROS-dependent damage in defined brain regions. Hum Mol Genet 21(23):5066–5077. https://doi.org/10.1093/hmg/dds350
Dillon PF, Clark JF (1990) The theory of diazymes and functional coupling of pyruvate kinase and creatine kinase. J Theor Biol 143(2):275–284
Dimer NW, Ferreira BK, Agostini JF, Gomes ML, Kist LW, Malgarin F, Carvalho-Silva M, Gomes LM, Rebelo J, Frederico MJS, Silva F, Rico EP, Bogo MR, Streck EL, Ferreira GC, Schuck PF (2018) Brain bioenergetics in rats with acute hyperphenylalaninemia. Neurochem Int 117:188–203. https://doi.org/10.1016/j.neuint.2018.01.001
Dyer CA (2000) Comments on the neuropathology of phenylketonuria. Eur J Pediatr 159(Suppl 2):S107–S108
Dzeja PP, Redfield MM, Burnett JC, Terzic A (2000) Failing energetics in failing hearts. Curr Cardiol Rep 2(3):212–217
Dzeja PP, Terzic A (1998) Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J 12(7):523–529
Dzeja PP, Vitkevicius KT, Redfield MM, Burnett JC, Terzic A (1999) Adenylate kinase-catalyzed phosphotransfer in the myocardium: increased contribution in heart failure. Circ Res 84(10):1137–1143
Fattal E, Rojas J, Youssef M, Couvreur P, Andremont A (1991) Liposome-entrapped ampicillin in the treatment of experimental murine listeriosis and salmonellosis. Antimicrob Agents Chemother 35(4):770–772
Feksa LR, Cornelio AR, Dutra-Filho CS, de Souza Wyse AT, Wajner M, Wannmacher CM (2003) Characterization of the inhibition of pyruvate kinase caused by phenylalanine and phenylpyruvate in rat brain cortex. Brain Res 968(2):199–205
Feksa LR, Cornelio AR, Rech VC, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2002) Alanine prevents the reduction of pyruvate kinase activity in brain cortex of rats subjected to chemically induced hyperphenylalaninemia. Neurochem Res 27(9):947–952
Fernandes CG, Leipnitz G, Seminotti B, Amaral AU, Zanatta A, Vargas CR, Dutra Filho CS, Wajner M (2010) Experimental evidence that phenylalanine provokes oxidative stress in hippocampus and cerebral cortex of developing rats. Cell Mol Neurobiol 30(2):317–326. https://doi.org/10.1007/s10571-009-9455-6
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153(1):23–36
Gray LR, Tompkins SC, Taylor EB (2014) Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci 71(14):2577–2604. https://doi.org/10.1007/s00018-013-1539-2
Grotto D, Santa Maria LD, Boeira S, Valentini J, Charao MF, Moro AM, Nascimento PC, Pomblum VJ, Garcia SC (2007) Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J Pharm Biomed Anal 43(2):619–624. https://doi.org/10.1016/j.jpba.2006.07.030
Gualano B, Roschel H, Lancha AH Jr, Brightbill CE, Rawson ES (2012) In sickness and in health: the widespread application of creatine supplementation. Amino Acids 43(2):519–529. https://doi.org/10.1007/s00726-011-1132-7
Gualano B, DESP V, Roschel H, Artioli GG, Neves M Jr, De SaPinto AL, Da Silva ME, Cunha MR, Otaduy MC, Leite Cda C, Ferreira JC, Pereira RM, Brum PC, Bonfa E, Lancha AH Jr (2011) Creatine in type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Med Sci Sports Exerc 43(5):770–778. https://doi.org/10.1249/MSS.0b013e3181fcee7d
Halliwell B, Gutteridge JM (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219(1):1–14
Hughes BP (1962) A method for the estimation of serum creatine kinase and its use in comparing creatine kinase and aldolase activity in normal and pathological sera. Clin Chim Acta 7:597–603
Huttenlocher PR (2000) The neuropathology of phenylketonuria: human and animal studies. Eur J Pediatr 159(Suppl 2):S102–S106
Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A (1999) Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res 85(4):357–363
Ishida T, Atobe K, Wang X, Kiwada H (2006) Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J Control Release 115(3):251–258. https://doi.org/10.1016/j.jconrel.2006.08.017
Janssen E, Dzeja PP, Oerlemans F, Simonetti AW, Heerschap A, de Haan A, Rush PS, Terjung RR, Wieringa B, Terzic A (2000) Adenylate kinase 1 gene deletion disrupts muscle energetic economy despite metabolic rearrangement. EMBO J 19(23):6371–6381. https://doi.org/10.1093/emboj/19.23.6371
Justo OR, Moraes AM (2005) Kanamycin incorporation in lipid vesicles prepared by ethanol injection designed for tuberculosis treatment. J Pharm Pharmacol 57(1):23–30. https://doi.org/10.1211/0022357055092
Kehrer JP (2000) The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 149(1):43–50
Kraft T, Hornemann T, Stolz M, Nier V, Wallimann T (2000) Coupling of creatine kinase to glycolytic enzymes at the sarcomeric I-band of skeletal muscle: a biochemical study in situ. J Muscle Res Cell Motil 21(7):691–703
Kreuter J (1995) Nanoparticulate systems in drug delivery and targeting. J Drug Target 3(3):171–173. https://doi.org/10.3109/10611869509015940
Kreuter J (2001) Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev 47(1):65–81
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5(2):227–231
Leong BK, Kociba RJ, Jersey GC (1981) A lifetime study of rats and mice exposed to vapors of bis(chloromethyl)ether. Toxicol Appl Pharmacol 58(2):269–281
Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, Javitt D, Kantrowitz J, Wall MM, Corcoran CM, Schobel SA, Small SA (2018) Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry 23(8):1764–1772. https://doi.org/10.1038/mp.2017.249
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474
Matsuzaki S, Szweda PA, Szweda LI, Humphries KM (2009) Regulated production of free radicals by the mitochondrial electron transport chain: cardiac ischemic preconditioning. Adv Drug Deliv Rev 61(14):1324–1331. https://doi.org/10.1016/j.addr.2009.05.008
McMorris T, Harris RC, Howard AN, Langridge G, Hall B, Corbett J, Dicks M, Hodgson C (2007) Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol Behav 90(1):21–28. https://doi.org/10.1016/j.physbeh.2006.08.024
Mezzomo NJ, Fontana BD, Müller TE, Duarte T, Quadros AA, Canzian J, Pompermaier A, Soares SM, Koakoski G, Loro VL, Rosemberg DB, Barcellos LJG (2019) Taurine modulates the stress response in zebrafish. Horm Behav 109:44–52. https://doi.org/10.1016/j.yhbeh.2019.02.006
Mitchell JJ, Trakadis YJ, Scriver CR (2011) Phenylalanine hydroxylase deficiency. Genet Med 13(8):697–707. https://doi.org/10.1097/GIM.0b013e3182141b48
Moraes TB, Dalazen GR, Jacques CE, de Freitas RS, Rosa AP, Dutra-Filho CS (2014) Glutathione metabolism enzymes in brain and liver of hyperphenylalaninemic rats and the effect of lipoic acid treatment. Metab Brain Dis 29(3):609–615. https://doi.org/10.1007/s11011-014-9491-x
Moraes TB, Jacques CE, Rosa AP, Dalazen GR, Terra M, Coelho JG, Dutra-Filho CS (2013) Role of catalase and superoxide dismutase activities on oxidative stress in the brain of a phenylketonuria animal model and the effect of lipoic acid. Cell Mol Neurobiol 33(2):253–260. https://doi.org/10.1007/s10571-012-9892-5
Moraes TB, Zanin F, da Rosa A, de Oliveira A, Coelho J, Petrillo F, Wajner M, Dutra-Filho CS (2010) Lipoic acid prevents oxidative stress in vitro and in vivo by an acute hyperphenylalaninemia chemically-induced in rat brain. J Neurol Sci 292(1–2):89–95. https://doi.org/10.1016/j.jns.2010.01.016
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Pena MJ, Pinto A, Daly A, MacDonald A, Azevedo L, Rocha JC, Borges N (2018) The use of glycomacropeptide in patients with phenylketonuria: a systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu10111794
Phaniendra A, Jestadi DB, Periyasamy L (2015) Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 30(1):11–26. https://doi.org/10.1007/s12291-014-0446-0
Poljsak B, Suput D, Milisav I (2013) Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013:956792. https://doi.org/10.1155/2013/956792
Preissler T, Bristot IJ, Costa BM, Fernandes EK, Rieger E, Bortoluzzi VT, de Franceschi ID, Dutra-Filho CS, Moreira JC, Wannmacher CM (2016) Phenylalanine induces oxidative stress and decreases the viability of rat astrocytes: possible relevance for the pathophysiology of neurodegeneration in phenylketonuria. Metab Brain Dis 31(3):529–537. https://doi.org/10.1007/s11011-015-9763-0
Radi R, Cassina A, Hodara R, Quijano C, Castro L (2002) Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med 33(11):1451–1464
Raha S, Robinson BH (2001) Mitochondria, oxygen free radicals, and apoptosis. Am J Med Genet 106(1):62–70. https://doi.org/10.1002/ajmg.1398
Rech VC, Feksa LR, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2002) Inhibition of the mitochondrial respiratory chain by phenylalanine in rat cerebral cortex. Neurochem Res 27(5):353–357
Rech VC, Mezzomo NJ, Athaydes GA, Feksa LR, Figueiredo VC, Kessler A, Franceschi ID, Wannmacher CMD (2018) Thiol/disulfide status regulates the activity of thiol-containing kinases related to energy homeostasis in rat kidney. An Acad Bras Cienc 90(1):99–108. https://doi.org/10.1590/0001-3765201720160348
Ribas GS, Sitta A, Wajner M, Vargas CR (2011) Oxidative stress in phenylketonuria: what is the evidence? Cell Mol Neurobiol 31(5):653–662. https://doi.org/10.1007/s10571-011-9693-2
Rojas DB, Gemelli T, de Andrade RB, Campos AG, Dutra-Filho CS, Wannmacher CMD (2012) Administration of histidine to female rats induces changes in oxidative status in cortex and hippocampus of the offspring. Neurochem Res 37(5):1031–1036. https://doi.org/10.1007/s11064-012-0703-7
Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray JM, Munnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228(1):35–51
Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T (2006) Cardiac system bioenergetics: metabolic basis of the Frank-Starling law. J Physiol 571(Pt 2):253–273. https://doi.org/10.1113/jphysiol.2005.101444
Saks V, Favier R, Guzun R, Schlattner U, Wallimann T (2006) Molecular system bioenergetics: regulation of substrate supply in response to heart energy demands. J Physiol 577(Pt 3):769–777. https://doi.org/10.1113/jphysiol.2006.120584
Sanayama Y, Nagasaka H, Takayanagi M, Ohura T, Sakamoto O, Ito T, Ishige-Wada M, Usui H, Yoshino M, Ohtake A, Yorifuji T, Tsukahara H, Hirayama S, Miida T, Fukui M, Okano Y (2011) Experimental evidence that phenylalanine is strongly associated to oxidative stress in adolescents and adults with phenylketonuria. Mol Genet Metab 103(3):220–225. https://doi.org/10.1016/j.ymgme.2011.03.019
Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S (2015) Advances and challenges of liposome assisted drug delivery. Front Pharmacol 6:286. https://doi.org/10.3389/fphar.2015.00286
Sharman R, Sullivan K, Young RM, McGill J (2012) Depressive symptoms in adolescents with early and continuously treated phenylketonuria: associations with phenylalanine and tyrosine levels. Gene 504(2):288–291. https://doi.org/10.1016/j.gene.2012.05.007
Singh B, Mundlamuri R, Friese T, Mundrigi A, Handt S, Loewe T (2018) Benchmarking of sterilizing-grade filter membranes with liposome filtration. PDA J Pharm Sci Technol 72(3):223–235. https://doi.org/10.5731/pdajpst.2017.007757
Sitta A, Barschak AG, Deon M, Barden AT, Biancini GB, Vargas PR, de Souza CF, Netto C, Wajner M, Vargas CR (2009) Effect of short- and long-term exposition to high phenylalanine blood levels on oxidative damage in phenylketonuric patients. Int J Dev Neurosci 27(3):243–247. https://doi.org/10.1016/j.ijdevneu.2009.01.001
Tarnopolsky MA (2007) Clinical use of creatine in neuromuscular and neurometabolic disorders. Subcell Biochem 46:183–204
Terjung RL, Clarkson P, Eichner ER, Greenhaff PL, Hespel PJ, Israel RG, Kraemer WJ, Meyer RA, Spriet LL, Tarnopolsky MA, Wagenmakers AJ, Williams MH (2000) American College of Sports Medicine roundtable. The physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc 32(3):706–717
Vieira Neto E, Maia Filho HS, Monteiro CB, Carvalho LM, da Cruz TS, de Barros BV, Ribeiro MG (2018) Behavioral and emotional problems in early-treated Brazilian children and adolescents with phenylketonuria. Med Sci Monit 24:7759–7769. https://doi.org/10.12659/MSM.909146
Wallace DC (1999) Mitochondrial diseases in man and mouse. Science 283(5407):1482–1488
Wallimann T (2007) Introduction–creatine: cheap ergogenic supplement with great potential for health and disease. Subcell Biochem 46:1–16
Wallimann T, Tokarska-Schlattner M, Schlattner U (2011) The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40(5):1271–1296. https://doi.org/10.1007/s00726-011-0877-3
Watanabe A, Kato N, Kato T (2002) Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci Res 42(4):279–285
Wohlfart S, Gelperina S, Kreuter J (2012) Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release 161(2):264–273. https://doi.org/10.1016/j.jconrel.2011.08.017
Zalba S, Navarro I, Troconiz IF, Tros de Ilarduya C, Garrido MJ (2012) Application of different methods to formulate PEG-liposomes of oxaliplatin: evaluation in vitro and in vivo. Eur J Pharm Biopharm 81(2):273–280. https://doi.org/10.1016/j.ejpb.2012.02.007
Acknowledgements
We would like to thank the Franciscan University (UFN) for the support and facilities and the financial support and fellowships from Coordination for the Improvement of Higher Education Personnel (CAPES—Finance Code 001), National Council for Scientific and Technological Development (CNPq), and Foundation for Research Support of the State of Rio Grande do Sul (Fapergs).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mezzomo, N.J., Becker Borin, D., Ianiski, F. et al. Creatine nanoliposome reverts the HPA-induced damage in complex II–III activity of the rats’ cerebral cortex. Mol Biol Rep 46, 5897–5908 (2019). https://doi.org/10.1007/s11033-019-05023-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05023-y