Abstract
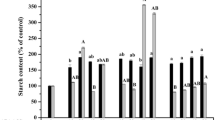
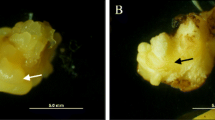
Somatic embryos of Larix × leptoeuropaea were grown on modified MSG media with 60 μM abscisic acid (ABA). These were compared to control embryos raised on the same medium without ABA. Transmission electron microscopy demonstrated that zonation of polyphenol production as well as presence of extracellular mucilage was markedly different in embryos raised with and without ABA. Idioblasts were found in subepidermal and pith regions of hypocotyls and among the subepidermal cells of cotyledons in embryos matured on ABA, but not in embryos matured without ABA. The embryonal root caps of ABA-treated embryos had substantial deposition of lipids and proteins in both the column and inner pericolumn regions, but not in the outer layer of the pericolumn. Control embryos showed no accumulation of proteins or lipids, but an increase in polyphenol accumulation, which had spread to the epidermal and sub-epidermal layers of the cotyledons and hypocotyl. Starch accumulation was similar over the course of development in embryos treated with or without ABA. Using gas chromatography-selected reaction monitoring mass spectrometry, it was shown that concentrations of ABA averaged 186 ± 17 μg g−1 dry weight (DW) in embryos raised on medium supplemented with this plant growth regulator, versus an average concentration of 55 ± 19 μg g−1 DW in embryos raised in the absence of ABA. No difference in ABA concentration was found between the root cap and the rest of ABA-treated embryos.
Similar content being viewed by others
References
Attree SM, Moore D, Sawhney VK & Fowke LC (1991) Enhanced maturation and desiccation tolerance of white spruce (Picea glauca (Moench) Voss) somatic embryos: effects of a non-plasmolysing water stress and abscisic acid. Ann. Bot. 68: 519–525
Attree SM, Pomeroy MK & Fowke LC (1992) Manipulation of conditions for the culture of somatic embryos of white spruce for improved triacylglycerol biosynthesis and desiccation tolerance. Planta 187: 395–404
Becwar MR, Nagmani R & Wann SR (1990) Initiation of embryogenic cultures and somatic embryo development in loblolly pine (Pinus taeda). Can. J. For. Res. 20: 810–817
Burrows GE & Stockey RA (1994) The developmental anatomy of cryptogeal germination in bunya pine (Araucaria bidwillii). Int. J. Plant Sci. 155: 519–537
Edlund A, Eklöf S, Sundberg B, Moritz T & Sandberg G (1995) A microscale technique for gas-chromatography mass-spectrometry measurements of picogram amounts of indole-3-acetic acid in plant tissues. Plant Physiol. 108: 1043–1047
Flinn BS, Roberts DR & Taylor IEP (1991) Evaluation of somatic embryos of interior spruce. Characterization and developmental regulation of storage proteins. Physiol. Plant. 82: 624–632
Gutmann M, von Aderkas P, Label P & Lelu M-A (1996) Effects of abscisic acid on somatic embryo maturation of hybrid larch. J. Exp. Bot. 47: 1905–1917
Hutchinson AH (1917) Morphology of Keteleeria fortunei.Bot. Gaz. 63: 124–134
Joy RW IV, Yeung YC, Kong L & Thorpe TA (1991) Development of white spruce somatic embryos: I. storage product deposition. In Vitro Cell Biol. 27P: 32–41
Klimaszewska K (1989) Plantlet development from immature zygotic embryos of hybrid larch through somatic embryogenesis. Plant Sci. 63: 95–103
Klimaszewska K & Smith DR (1997) Maturation of somatic embryos of Pinus strobus is promoted by a high concentration of gellan gum. Physiol. Plant. 100: 949–957
Kong L, Attree SM & Fowke LC (1997) Changes of endogenous hormone levels in developing seeds, zygotic embryos and megagametophytes in Picea glauca. Physiol. Plant. 101: 23–30
Label P & Lelu M-A (2000) Exogenous abscisic acid fate during maturation of hybrid larch (Larix × leptoeuropaea) somatic embryos. Physiol. Plant. 109: 456–462
Lelu M-A & Label P (1994) Changes in the levels of abscisic acid and its glucose ester conjugate during maturation of hybrid. larch (Larix × leptoeuropaea) somatic embryos, in relation to germination and plantlet recovery. Physiol. Plant. 92: 53–60
Lelu M-A, Bastien C, Klimazewska K, Ward C & Charest PJ (1994a) An improved method for somatic plant production in hybrid larch (Larix × leptoeuropaea): Part I. Somatic embryo maturation. Plant Cell Tissue. Org. Cult. 36: 107–115
Lelu M-A, Klimazewska K & Charest PJ (1994b) Somatic embryogenesis from immature and mature zygotic embryos and from cotyledons and needles of somatic plantlets of Larix.Can.J.For. Res. 24: 100–106
Lelu M-A, Klimazewska K, Pflaum G & Bastien C (1995) Effect of maturation duration on desiccation tolerance in hybrid larch (Larix × leptoeuropaea Dengler) somatic embryos. In Vitro Cell Dev. Biol. 31: 15–20
Roberts DR, Flinn BS, Webb DT, Webster FB & Sutton BCS (1990) Abscisic acid and indole-3-butyric acid regulation of maturation and accumulation of storage proteins in somatic embryos of interior spruce. Physiol. Plant. 78: 355–360
Rohr R, von Aderkas P & Bonga JM (1989) Ultrastructural changes in haploid embryoids of Larix decidua during early embryogenesis. Am. J. Bot. 76: 460–1467
Schopf JM (1943) The embryology of Larix. Ill. Biol. Monographs 19: 1–97
Singh H (1978) Embryology of Gymnosperms. Gebrüder Borntraeger, Berlin
Tautorus T, Fowke LC & Dunstan DI (1991) Somatic embryogenesis in conifers. Can. J. Bot. 69: 1873–1899
Thiery JP (1967) Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J. Microsc. 6: 987–1017
Tremblay L & Tremblay FM (1995) Maturation of black spruce somatic embryos: sucrose hydrolysis and resulting osmotic pressure of the medium. Plant Cell Tiss. Org. Cult. 4: 9–46
von Aderkas P, Bonga J, Klimaszewska K & Owens J (1991) Comparison of larch embryogeny in vivo and in vitro. In: Ahuja MR (ed.) Woody Plant Biotechnology (pp 139–155) Plenum Press, New York
von Aderkas P, Lelu M-A & Label P (2001) Plant growth regulator levels during maturation of larch somatic embryos. Plant Physiol. Biochem. 39: 495–502
von Arnold S & Hakman I (1988) Regulation of somatic embryo development in Picea abies by absicisic acid (ABA). J Plant Physiol. 132: 164–169
von Guttenberg H (1961) Grundzüge der Histogenese höherer Pflanzen. II. Die Gymnospermen. Gebrüder Borntraeger, Berlin
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
von Aderkas, P., Rohr, R., Sundberg, B. et al. Abscisic acid and its influence on development of the embryonal root cap, storage product and secondary metabolite accumulation in hybrid larch somatic embryos. Plant Cell, Tissue and Organ Culture 69, 111–120 (2002). https://doi.org/10.1023/A:1015245627220
Issue Date:
DOI: https://doi.org/10.1023/A:1015245627220