Abstract
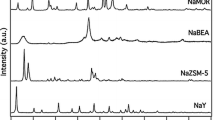
Proton transfer reactions between zeolite Y surface and 1-naphthylamine (NA) in the ground and excited states have been studied by laser-induced picosecond spectroscopy. The acidic form of zeolite Y readily protonates NA in the ground state. At low acid strength of the zeolite the excited state of the protonated NA transfers back the proton to the zeolite surface as indicated by the fluorescence spectra. At high acid strength of the zeolite the fluorescence comes from the protonated form of NA and from an adduct “X” previously found in highly concentrated HClO4 solutions. The concentrations of the protonated NA and X increase with the reduction in the unit cell size. The presence of these species is discussed in terms of the next nearest neighbor “NNN” theory of zeolite Y acidity and the role of the non-framework aluminum. The acidity of the zeolite is estimated, based on the fluorescence lifetimes of X, to vary from 3.7 to 17 M HClO4 depending on the unit cell size. Low loading levels of NA in the zeolite pores are best in studying the proton transfer reaction and for the estimation of the surface acidity of zeolite Y.
Similar content being viewed by others
References
R.A. van Santen and G.J. Kramer, Chem. Rev. 95 (1995) 637.
A. Corma, Chem. Rev. 97 (1997) 2373.
E.W. Hansen, F. Courivaud, A. Carlson, S. Kolboe and M. Stocker, Micropor. Mesopor. Mater. 22 (1997) 309.
S. Biz and M.L. Occelli, Catal. Rev. Sci. Eng. 40 (1998) 329.
Mirodatus and D. Bathomeuf, J. Catal. 93 (1985) 246.
A. Corma, H. Garcia, S. Iborra and J. Primo, J. Catal. 120 (1989) 78.
C.V. McDaniel and P.J. Maher, in: Conference on Molecular Sieves (Society of Chemical Industry, London, 1967) p. 186.
G.T. Kerr, in: Molecular Sieves, Adv. Chem. Ser., Vol. 121, eds. W.M. Meier and J.B. Uytterhoeven (1973) p. 219.
P.A. Jalil, M.A. Al-Daous, A.A. Al-Arfaj, A.M. Al-Amer, J. Beltramini and S.A.I. Barri, Appl. Catal. A 207 (2001) 159.
G.T. Kerr, J. Phys. Chem. 73 (1969) 2780.
G.W. Skeels and E.M. Flanigen, in: Zeolite Synthesis, Adv. Chem. Ser., Vol. 435 (1989) p. 420.
L.A. Pine, P.J. Maher and W.A. Wachter, J. Catal. 85 (1984) 466.
A.A. Eremenko, F.M. Bobonitz, M.V. Cost, M.A. Pionkovskaya, M.Yu. Sakhnovskii and I.E. Niemark, Opt. Spektrosk. 35 (1973) 224.
B.H. Bartez and N.J. Turro, J. Photochem. 24 (1984) 201.
P. Hite, R. Krasnansky and J.K. Thomas, J. Phys. Chem. 90 (1986) 5295.
J.K. Thomas, J. Phys. Chem. 91 (1987) 267.
J.K. Thomas, Chem. Rev. 93 (1993) 301.
X. Liu, K.K. Iu and J.K. Thomas, J. Phys. Chem. 93 (1989) 4120.
X. Liu, K.K. Iu and J.K. Thomas, J. Phys. Chem. 98 (1994) 7877.
B.H. Milosavljevic and J.K. Thomas, J. Phys. Chem. 92 (1988) 2997.
H. Shizuka, Acc. Chem. Res. 18 (1985) 141.
A. Corma, M.S. Grande, V. Gonzales Alfaro and A.V. Orchilles, J. Catal. 159 (1996) 375.
S.A. Rutten and J.K. Thomas, J. Phys. Chem. B 102 (1998) 598.
N.J. Turro, Pure Appl. Chem. 58 (1986) 1219.
V. Ramamurthy, J. Am. Chem. Soc. 116 (1994) 1345.
J.C. Scaiano, N.C. De Locas, J. Andraos and H. Garcia, Chem. Phys. Lett. 233 (1995) 5.
S. Corrent, P. Hahn, G. Pohlers, T.G. Connoly, G.C. Scaiano and H. Garcia, J. Phys. Chem. B 102 (1998) 5852.
M. Alvaro, H. Garcia, S. Corrent and J.C. Scaiano, J. Phys. Chem. B 102 (1998) 7530.
I.F.J. Vankelecom, D. Depre, S.D. Beukelaeur and J.B. Uytterhoeven, J. Phys. Chem. B 99 (1995) 13193.
I.F.J. Vankelecom, C. Dotremont, M. Morbe and J.B. Uytterhoeven, J. Phys. Chem. B 101 (1997) 2154.
C. Dotremont, I.F.J. Vankelecom, M. Morbe and J.B. Uytterhoeven, J. Phys. Chem. B 101 (1997) 2160.
A.A. El-Rayyes, H.P. Perzanowski, S.A.I. Barri and U.K.A. Klein, J. Phys. Chem., accepted.
D.W. Breck and E.M. Flanigen, in: Conference on Molecular Sieves (Society of Chemical Industry, London, 1967) p. 47.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
El-Rayyes, A.A., Perzanowski, H., Klein, U.K. et al. Acidity of Zeolite Y—Probed by Adsorption of 1-Naphthylamine and Studied by Laser-Induced Fluorescence Spectroscopy. Catalysis Letters 78, 161–170 (2002). https://doi.org/10.1023/A:1014937724766
Issue Date:
DOI: https://doi.org/10.1023/A:1014937724766