Abstract
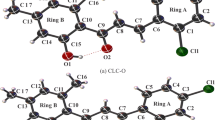
The results of a nonempirical calculation of the 2,2,2,4,4,4-hexachloro-1,3-dimethyl-1,3-diaza-2,4-diphosphetane (Cl3PNCH3)2 molecule by the RHF 6-31G(d) method are in agreement with the data of X-ray structural analysis of this compound. Calculated 35Cl NQR frequencies for axial and equatorial chlorine atoms are close to the experimental values. The population of the orbitals of the lone electron pairs and the p σ orbitals of the equatorial Cl atoms were significantly lower than those of the axial atoms. Among the MO there was no MO corresponding to a three-center bond involving a P atom and axial Cl and N atoms.
Similar content being viewed by others
REFERENCES
V. P. Feshin, G. V. Dolgushin, M. G. Voronkov, B. V. Timokhin, V. K. Dmitriev, V. I. Dmitriev, V. N. Vengel′nikova, Yu. E. Sapozhnikov, and Ya. B. Yasman, Dokl. Akad. Nauk SSSR, 261, 436 (1981).
V. P. Feshin, V. P. Elin, B. V. Timokhin, V. K. Dmitriev, G. V. Dolgushin, A. V. Kalabina, and M. G. Voronkov, Dokl. Akad. Nauk SSSR, 290, 1423 (1986).
V. P. Feshin and M. Yu. Kon′shin, Zh. Obshch. Khim., 66, 948 (1996).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, P. M. W. Gill, B. G. Johnson, M. A. Robb, J. R. Cheeseman, T. Keith, G. A. Petersson, J. A. Montgomery, K. Raghavachari, M. A. Al-Laham, V. G. Zakrzewski, J. V. Ortiz, J. B. Foresman, J. Cioslowski, B. B. Stefanov, A. Nanayakkara, M. Challacombe, C. Y. Peng, P. Y. Ayala, W. Chen, M. W. Wong, J. L. Andres, E. S. Replogle, R. Gomperts, R. L. Martin, D. J. Fox, J. S. Binkley, D. J. Defrees, J. Baker, J. P. Stewart, M. Head-Gordon, C. Gonzalez, and J. A. Pople, GAUSSIAN 94, Revision E. 3, Gaussian Inc., Pittsburgh PA (1995).
H. Hess and D. Forst, Z. Anorg. Allgem. Chem., 342, 240 (1966).
L. G. Hoard and R. A. Jacobson, J. Chem. Soc., A, 1203 (1966).
R. Keat, A. L. Porte, D. A. Tong, and R. A. Shaw, J. Chem. Soc., Dalton Trans., 1648 (1972).
A. D. Gordeev, E. S. Kozlov, and G. B. Soifer, Zh. Strukt. Khim., 14, 934 (1973).
V. P. Feshin, Electron Effects in Organic and Heteroorganic Molecules [in Russian], Ural. Otd. RAN, Ekaterinburg (1997), 377 pp.
V. P. Feshin and E. V. Feshina, Z. Naturforsch., 55a, 555 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Feshin, V.P., Soifer, G.B. Special Features of the Geometry of the 2,2,2,4,4,4-Hexachloro-1,3-dimethyl-1,3-diaza-2,4-diphosphetane Molecule and Its Electron Distribution According to Data of ab initio Calculations. Chemistry of Heterocyclic Compounds 38, 106–109 (2002). https://doi.org/10.1023/A:1014867612497
Issue Date:
DOI: https://doi.org/10.1023/A:1014867612497