Abstract
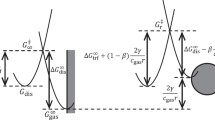
Effect of the activity of the catalyst in gas-generating porous electrodes (GPE) of the DSA type on the anodic branch of polarization curves is studied. The structure of the GPE porous space and the electrode kinetics are assumed to remain invariant. The quantities that are varied are the electrode thickness and the exchange current j 0. Characteristics of GPE electrodes of high, medium, and low activity (j 0 of 1.2 × 10–2,1.2 × 10–4, and 1.2 × 10–6 A cm–2, respectively) are compared. The standard thicknesses, calculated for these electrodes, are 1, 5, and 20 μm. In GPE of high and medium activity, after the emergence of first gas pores, the gas generation region, which before this was localized in a narrow region near the front surface of GPE, can rapidly expand by tens of times, so can the overall current. With a further overvoltage increase, ohmic restrictions again press the gas generation region to the front surface of GPE. In the low-activity GPE, the expansion of the gas generation region and the increase in the overall current are insignificant. It is shown that the medium-activity GPE commonly used in the chlorine electrolysis may be viewed as optimal. However, this by no means implies that the DSA operation mechanism is ideal. Perfecting the porous-space structure of DSA even further gives one a chance to considerably better its electrochemical characteristics.
Similar content being viewed by others
REFERENCES
Yakimenko, L.M., Proizvodstvo khlora, kausticheskoi sody i neorganicheskikh khlorproduktov (Production of Chlorine, Sodium Hydroxide, and Inorganic Chlorine Derivatives), Moscow: Khimiya, 1974.
Yakimenko, L.M. and Pasmanik, M.I., Spravochnik po proizvodstvu khlora, kausticheskoi sody i osnovnykh khlorproduktov (Handbook on the Production of Chlorine, Sodium Hydroxide, and Basic Chlorine Derivatives), Moscow: Khimiya, 1976.
Yakimenko, L.M., Elektrodnye materialy v prikladnoi elektrokhimii (Electrode Materials in Applied Electrochemistry), Moscow: Khimiya, 1977.
Abstracts of Papers, IV Vsesoyuz. seminar “Maloiznashivaemye anody i ikh primenenie v elektrokhimicheskikh protsessakh” (IV All-Union Workshop on Nonsacrificial Anodes and Their Application in Electrochemical Processes), Moscow: NIITeKhim, 1979.
Yakimenko, L.M., Poluchenie vodoroda, kisloroda, khlora i shchelochei (Production of Hydrogen, Oxygen, Chlorine, and Alkalis), Moscow: Khimiya, 1981.
Fioshin, M.Ya. and Smirnova, M.G., Tekhnika elektroliza (The Electrolysis), Rostov-on-Don: Rostov. Univ., 1983.
Abstracts of Papers, V Vsesoyuz. seminar “Maloiznashivaemye anody i ikh primenenie v elektrokhimicheskikh protsessakh” (V All-Union Workshop on Nonsacrificial Anodes and Their Application in Electrochemical Processes), Moscow, 1984.
Abstracts of Papers, VI Vsesoyuz. seminar “Maloiznashivaemye anody i ikh primenenie v elektrokhimicheskikh protsessakh” (VI All-Union Workshop on Nonsacrificial Anodes and Their Application in Electrochemical Processes), Moscow, 1987.
Yakimenko, L.M., Modylevskaya, I.D., and Tkachek, Z.A., Elektroliz vody (Electrolysis of Water), Moscow: Khimiya, 1970.
Kryukov, Yu.I., Chernyshov, S.F., and Pshenichnikov, A.G., Hydrogen Energy Progress, VII, Veziroglu, T.N., et al., Eds., New York: Pergamon, 1988, vol. 1, p. 403.
Pshenichnikov, A.G., Kazarinov, V.E., and Naumov, I.P., Elektrokhimiya, 1991, vol. 27, p. 1555.
Chernyshev, S.F., Kryukov, Yu.I., and Pshenichnikov, A.G., Elektrokhimiya, 1992, vol. 28, p. 391.
Pecherskii, M.M., Gorodetskii, V.V., Evdokimov, S.V., and Losev, V.V., Elektrokhimiya, 1981, vol. 17, p. 1087.
Chirkov, Yu.G., Elektrokhimiya, 2000, vol. 36, p. 526.
Chirkov, Yu.G. and Rostokin, V.I., Elektrokhimiya, 2000, vol. 36, p. 735.
Chirkov, Yu.G. and Rostokin, V.I., Elektrokhimiya, 2001, vol. 37, p. 336.
Chirkov, Yu.G. and Rostokin, V.I., Elektrokhimiya, 2001, vol. 37, p. 409.
Chirkov, Yu.G. and Chernenko, A.A., Elektrokhimiya, 2001, vol. 37, p. 546.
Chirkov, Yu.G. and Rostokin, V.I., Elektrokhimiya, 2001, vol. 37, p. 557.
Chirkov, Yu.G. and Rostokin, V.I., Elektrokhimiya, 2001, vol. 37, p. 987.
Chirkov, Yu.G. and Rostokin, V.I., Elektrokhimiya, 2001, vol. 37, p. 1107.
Chirkov, Yu.G. and Rostokin, V.I., Elektrokhimiya, 2002, vol. 38, p. 316.
Erenburg, R.G. and Krishtalik, L.I., Elektrokhimiya, 1987, vol. 23, p. 8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chirkov, Y.G., Rostokin, V.I. Gas-generating Porous Electrodes: Effect of the Catalyst Activity on the Polarization Curves. Russian Journal of Electrochemistry 38, 285–292 (2002). https://doi.org/10.1023/A:1014738924888
Issue Date:
DOI: https://doi.org/10.1023/A:1014738924888