Abstract
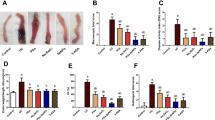
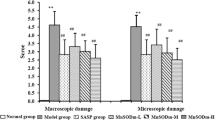
Free radicals play an important role in the initiation and progression of inflammatory bowel disease (IBD). Therefore, the reduction or elimination of adverse oxidant effects can provide novel therapy for IBD. Here, the antioxidant capacity and protective effects of a new class of chemically modified hetastarch (polynitroxyl starch, or PNS) plus 4-hydroxyl-2,2,6,6-tetramethylpiperidine-N-oxyl (Tempol or TPL) (PNS/TPL) were assessed in a model of colitis. The superoxide scavenging capacity of PNS/TPL—that is, the inhibition of the reduction of cytochrome c in the presence of xanthine/xanthine oxidase (X/XO)—was evaluated in vitro. The effects of PNS/TPL on X/XO–induced neutrophil endothelial adhesion in vitro were investigated. Also, this study tested the protection produced by PNS/TPL in a mouse model of trinitrobenzene sulfonic acid (TNBS)–induced colitis. PNS/TPL was given intravenously immediately before (<30 min) and intraperitoneally at 24 and 72 hr after TNBS induction. The body weight and survival rate of the mice were checked daily. Colonic mucosal damage was assessed on the 7th day by measuring intestinal permeability to Evans blue (EB) in vivo. The ability of PNS to reoxidize bioreduced TPL was documented by whole-body electron paramagnetic resonance (EPR) detection. We found that PNS or TPL exhibits superoxide dismutase (SOD)–like activity, with approximately 2% of SOD activity occurring on a molar basis. The endothelial–neutrophil adherence induced by X/XO was significantly inhibited by PNS/TPL but not by TPL alone. PNS/TPL protected against cachexia and mortality, both usually induced by TNBS. Epithelial permeability was increased significantly in TNBS mice but was ameliorated by the administration of PNS/TPL. In conclusion, PNS/TPL may be beneficial in the treatment or prevention of IBD through its antioxidant effects, which inhibit oxidant-mediated leukocyte adhesion and injury to endothelial cells.
Similar content being viewed by others
REFERENCES
Schreiber, S., A. Raedler, W. F. Stenson, and R. P. MacDermott. 1992. The role of the mucosal immune system in inflammatory bowel disease. Gastroenterol. Clin. North Am. 21:451–502.
Grisham, M. B. and D. N. Granger. 1998. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig. Dis. Sci. 33:6S–15S.
Grisham, M. B. 1994. Oxidants and free radicals in inflammatory bowel disease. Lancet 344:859–861.
Klebanoff, S. J. 1992. Oxygen metabolites from phagocytes. In J. I. Gallin, I. M. Goldstein, and R. Snyderman, eds. Inflammation: Basic principles and clinical correlates1. New York: Raven Press, 541–588.
Harris, M. L., H. J. Schiller, P. M. Reilly, M. Donowitz, M. B. Grisham, and G. B. Bulkley. 1992. Free radicals and other reactive oxygen metabolites in inflammatory bowel disease. Cause, consequence, or epiphenomenon? Pharmacol. Ther. 53:375–408.
Grisham, M. B. 1993. Role of reactive oxygen metabolites in inflammatory bowel disease. Curr. Opin. Gastroenterol. 9:971–980.
Hahn, S. M., C. M. Krishna, A. Samuni, J. B. Mitchell, and A. Russo. 1991. Mn (III)-desferrioxamine superoxide dismutasemimic: Alternative modes of action. Arch. Biochem. Biophys. 288:215–219.
Rabinowitch, H. D., G. M. Rosen, and I. Fridovich. 1989. A mimic of superoxide dismutase activity protects Chlorella sorokiniana against the toxicity of sulfate. Free Radic. Biol. Med. 6:45–48.
Beyer, W. F. and I. Fridovich. 1989. Characterization of a superoxide dismutase mimic prepared from desferrioxamine and MnO2. Arch. Biochem. Biophys. 15: 271:149–156.
Nagano, T., T. Hirano, and M. Hirobe. 1989. Superoxide dismutase mimics based on iron in vivo. J. Biol. Chem. 264:9243–9249.
Huber, K. R., R. Sridhar, E. H. Griffith, E. L. Amma, and J. Roberts. 1987. Superoxide dismutase-like activities of copper(II) complexes tested in serum. Biochim. Biophys. Acta 915:267–276.
Samuni, A., C. M. Krishna, P. Riesz, E. Finkelstein, and A. Russo. 1988. A novel metal-free low molecular weight superoxide dismutase mimic. J. Biol. Chem. 263:17921–17924.
Weiss, R. H., A. G. Flickinger, W. J. Rivers, M. M. Hardy, K. W. Aston, U. S. Ryan, and D. P. Riley. 1993. Evaluation of activity of putative superoxide dismutase mimics. Direct analysis by stopped-flow kinetics. J. Biol. Chem. 268:23049–23054.
Krishna, M. C. and A. Samuni. 1994. Nitroxides as antioxidants. Meth. Enzymol. 234:580–589.
Mitchell, J. B., A. Samuni, M. C. Krishna, W. G. Degraff, M. S. Ahn, U. Samuni, and A. Russo. 1990. Biologically active metal-independent superoxide dismutase mimics. Biochemistry 29:2802–2807.
Samuni, A., D. Winkelsberg, A. Pinson, S. M. Hahn, J. B. Mitchell, and A. Russo. 1991. Nitroxide stable radicals protect beating cardiomyocytes against oxidative damage. J. Clin. Invest. 87:1526–1530.
Samuni, A., D. Godinger, J. Aronovitch, A. Russo, and J. B. Mitchell. 1991. Nitroxides block DNA scission and protect cells from oxidative damage. Biochemistry 30:555–561.
Mohsen, M., A. Pinson, R. Zhang, and A. Samuni. 1995. Do nitroxides protect cradiomyoctes from hydrogen peroxide or superoxide? Mol. Cell. Biochem. 145:103–110.
Beit-Yannai, E., R. Zhang, V. Trembovler, A. Samuni, and E. Shohami. 1996. Cerebroprotective effect of stable nitroxide radicals in closed head injury in the rat. Brain Res. 717:22–28.
Samuni, A., J. B. Mitchell, W. DeGraff, C. M. Krishma, U. Samuni, and A. Russo. 1991. Nitroxide SOD-mimics: Modes of action. Free Radic. Res. Commun. 12-13(pt. 1):187–194.
Rachmilewitz, D., F. Karmeli, E. Okon, and A. Samuni. 1994. A novel antiulcergenic stable radical prevents gastric mucosal lesions in rats. Gut 35:1181–1188.
Karmeli, F., R. Eliakim, E. Okon, A. Samuni, and D. Rachmilewitz. 1995. A stable nitroxide radical effectively decreases mucosal damage in experimental colitis. Gut 37:386–393.
Kuppusamy, P., P. Wang, J. L. Zweier, M. C. Krishna, J. B. Mitchell, L. Ma, C. E. Trimble, and C. J. C. Hsia. 1996. Electron paramagnetic resonance imaging of rat heart with nitroxide and polynitroxyl-albumin. Biochemistry 35:7051–7057.
Russell, J., N. Okayama, J. S. Alexander, D. N. Granger, and C. J. C. Hsia. 1998. Pretreatment with polynitroxyl albumin (PNA) inhibits ischemia-reperfusion induced leukocyte-endothelial cell adhesion. Free Radic. Biol. Med. 25:153–159.
Okayama, N., J. H. Park, L. Coe, D. N. Granger, L. Ma, C. J. Hisa, and J. S. Alexander. 1999. Polynitroxyl alpha-alpha-hemoglobin (PNH) inhibits peroxide-and superoxide-mediated neutrophil adherence to human endothelial cells. Free Radic. Res. 31(pt. 1):53–58.
Treib, J., J. F. Baron, M. T. Grauer, and R. G. Strauss. 1999. An international view of hydroxyethyl starches. Intensive Care Med. 25:258–268.
Saito, K., H. Yoshioka, N. Ito, S. Kazama, H. Tanizawa, Y. Lin, H. Watanabe, T. Ogata, and H. Kamada. 1997. Spatiotemporal ESR-CT study on the metabolism of spin-labeled polysaccharide in a mouse. Biol. Pharm. Bull. 20:904–909.
Tateishi, H., K. Mitsuyama, A. Toyonaga, M. Tomoyose, and K. Tanikawa. 1997. Role of cytokines in experimental colitis: Relation to intestinal permeability. Digestion 58:271–281.
Crapo, J. D., J. M. McCord, and I. Fridovich, I. 1978. Preparation and assay of superoxide dismutases. Methods Enzymol. 53:382–393.
McCord, J. and I. Fridovich. 1969. Superoxide dismutases. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244:6049–6055.
Fridovich, I. 1989. Superoxide dismutases. An adaption to a paramagnetic gas. J. Biol. Chem. 264:7761-7764.
Boccu, E., G. P. Velo, and F. M. Veronese. 1982. Pharmacokinetic properties of polyethylene glycol derivatized superoxide dismutase. Pharmacol. Res. Commun. 14:113–120.
Veronese, F. M., E. Boccu, O. Schiavon, G. P. Velo, A. Conforti, L. Franco, and R. Milanino. 1983. Anti-inflammatory and pharmacokinetic properties of superoxide dismutase derivatized with polyethylene glycol via active esters. J. Pharm. Pharmacol. 35:757–758.
Miura, Y., H. Utsumi, and A. Hamada. 1993. Antioxidant activity of nitroxide radicals in lipid peroxidation of rat liver microsomes. Arch. Biochem. Biophys. 300:148–156.
Oz, M. C., B. A. Zikria, P. F. McLeod, and S. J. Popilkis. 1991. Hydroxyethyl starch macromolecules and superoxide dismutase effects on myocardial reperfusion injury. Am. J. Surg. 162:59–62.
Schell, R. M., D. J. Cole, R. L. Schultz, and T. N. Osborne. 1992. Temporary cerebral ischemia, effects of pentastarch or albumin on reperfusion injury. Anesthesiology 77:86–92.
Oz, M. C., M. F. FitzPatrick, B. A. Zirka, D. J. Pinsky, and W. N. Duran. 1995. Attenuation of microvascular permeability dysfunction in postischemic striated muscle by hydroxyethyl starch. Microvasc. Res. 50:71–79.
Willms, C. D., I. J. Dawidson, J. M. Armstrong, M. Kwon, R. Risser, Z. F. Sandor, and J. T. Sentementes. Pentafraction-Du Pont versus albumin for resuscitation of a lethal intestinal ischemic shock in rats. Circ. Shock 33:216-221.
Pieper, G. M., G. J. Gross, and B. Kalyanaraman. 1990. An ESR study of the nitroxide radical of pentastarch-conjugated deferoxamine. Free Radic. Biol. Med. 9:211–218.
Keshavarzian, A., S. Sedghi, J. Kanofsky, T. List, C. Robinson, C. Ibrahim, and D. Winship. 1992. Excessive production of reactive oxyten metabolites by inflamed colon: Analysis by chemiluminescence probe. Gastroenterology 103:177–185.
Simmonds, N. J., R. E. Allen, T. R. Stevens, R. N. Someren, D. R. Blake, and D. S. Rampton. 1992. 103:186–96.
Oshitani, N., A. Kitano, H. Okabe, S. Nakamura, T. Matsumato, and K. Kobayashi. 1993. Location of superoxide anion generation in human colonic mucosa obtained by biopsy. Gut 34:936–938.
Emerit, J., S. Pelletier, D. Tosoni-Verilgnue, and M. Mollet. 1989. Phase II trial of copper ?zinc superoxide dismutase (Cu ?Zn SOD) in treatment of Crohn's disease. Free Radic. Biol. Med. 7:145–149.
Keshavarzian, A., G. Morgan, S. Sedghi, and J. H. Gordon. 1990. Role of reactive oxygen metabolites in experimental colitis. Gut 31:786–790.
Keshavarzian, A., J. Haydeck, R. Zahihi, M. Doria, M. D'Astice, and J. R. J. Sorenson. 1992. Agents capable of eliminating reactive oxygen species catalase, WR-2721 or Cu (II)2(3,5-DIPS)4 decreases experimental colitis. Dig. Dis. Sci. 37:1866–1873.
Hahn, S. M., F. J. Sullivan, A. M. DeLuca, M. C. Krishna, N. Wersto, D. Venzon, A. Russo, and J. B. Mitchell. 1997. Evaluation of Tempol radioprotection in a murine tumor model. Free Radic. Biol. Med. 22:1211–1216.
Fuchs, J., H. J. Freisledben, M. Podda, G. Zimmer, R. Milbradt, and L. Packer. 1993. Nitroxide radical biostability in skin. Free Radic. Biol. Med. 15:415–423.
Chen, K. and H. M. Swartz. 1988. Oxidation of hydroxylamines to nitroxide spin labels in living cells. Biochim. Biophys. Acta. 970:270–277.
Suzuki, M., M. B. Grisham, and D. N. Granger. 1994. Leukocyte- endothelial cell adhesive interactions: Role of xanthine oxidase- derived oxidants. J. Leuk. Biol. 50:488–494.
Ichikawa, H., S. Flores, P. R. Kvietys, R. E. Wolf, T. Yoshikawa, D. N. Granger, and T. Y. Aw. 1997. Molecular mechanisms of anoxia ?reoxygenation-induced neutrophil adherence to cultured endothelial cells. Cir. Res. 81:922–923.
Bjarnason, I., A. Macpherson, and D. Hollander. 1995. Intestinal permeability: An overview. Gastroenterology 108:1566–1581.
Saria, A. and J. M. Lundberg. 1983. Evans blue fluorescence: Quantitative and morphological evaluation of vascular permeability in animal tissues. J. Neurosci. Meth. 8:41–49.
Lange, S., D. S. Delbro, and E. Jennisehe. Evans blue permeation of intestinal mucosa in rat. Scand. J. Gastroenterol. 29:38-46.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Park, J.H., Ma, L., Oshima, T. et al. Polynitroxylated Starch/TPL Attenuates Cachexia and Increased Epithelial Permeability Associated with TNBS Colitis. Inflammation 26, 1–11 (2002). https://doi.org/10.1023/A:1014420327417
Issue Date:
DOI: https://doi.org/10.1023/A:1014420327417