Abstract
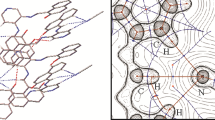
N-(3-pyridil)-2-oxo-1-naphthylidenemethylamine (C16H12N2O) was studied by elemental analysis, IR, 1H NMR, and UV–visible techniques and X-ray diffraction methods. The UV–visible spectrum of the compound was investigated in solutions effect polarity. The polarity of the some solvents was modifierly the additional (CF3COOH) and [(C2H5)3N]. The compound is in tautomeric equilibrium (phenol-imine O–H···N and keto-amine O···H–N forms) in polar and nonpolar solvents. The keto-amine form is observed in basic solutions of DMSO, ethanol, chloroform, benzene, cyclohexane, and in acidic solutions of chloroform and benzene, but not in acidic solutions of DMSO and ethanol. The compound crystallizes in the monoclinic, space group P21/a with a = 7.010(5) Å, b = 13.669(4) Å, c = 12.764(4) Å, β = 101.23(4)°, V = 1199.6(10) Å3, Z = 4, D c = 1.375 g/cm3, μ(Mo Kα) = 0.088 mm−1, R = 0.045 for 1658 reflections [I > 2σ(I)]. The title compound is not planar two Schiff base moieties A [C1–C11, O1] and B [N1, C12, C13, N2, C14, C15, C16] are inclined at an angle of 27.4(1)° reflecting mainly the twist about C12–N1 [C11–C12–N1–C13, 29.7(2)°]. There is a strong intramolecular hydrogen bond (O–H···N) of 2.529(2) Å.
Similar content being viewed by others
References
Yýldýz, M.; Kýlý¸c, Z.; Hökelek, T. J. Mol. Struct. 1998, 441, 1.
Nazýr, H.; Yýldýz, M.; Yýlmaz, H.; Tahir, M.N.; Ñlkü, D. J. Mol. Struct. 2000, 524, 241.
Costamagna, J.; Vargas, J.; Latorre, R.; Alvarado, A.; Mena, G.; Coord. Chem. Rev. 1992, 119, 67.
Gavranic, M.; Kaitner, B.; Mestrovic, E. J. Chem. Crystallogr. 1996, 26, 23.
Salman, S.R.; Shawkat, S.H.; Al-Obaidi, G.M. Can. J. Spect. 1990, 35, 25.
Molecular Structure Corporation. MSC/AFC Diffractometer Control Software: MSC 3200 Research Forest Drive; Molecular Structure Corporation: The Woodlands, TX, 1994.
Molecular Structure Corporation.TEXSANforWindowsVersion 1.03. Single Crystal Structure Analysis Software: MSC 3200 Research Forest Drive; Moluclar Structure Corporation: The Woodlands, TX, 1997.
Sheldrick, G.M. SHELXS-97, Programfor the Solution of Crystal Structures; University of Gottingen: Germany, 1997.
Sheldrick, G.M. SHELXL-97, Program for the Refinement of Crystal Structures, University of Gottingen: Germany, 1997.
Farrugia, L.J. J. Appl.Crystallogr. 1997, 30, 565.
Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. J. Cem. Soc., Perkin Trans II 1987, S1-S19.
Kaitner, B.; Pavlovic, G. Acta Crystallogr. Sect. C 1996, 52, 2573.
Elmali, A.; Kabak, M.; Kavlakoğlu, E.; Elerman,Y.; Durlu, T.N. J. Mol. Struct. 1999, 510, 207.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ünver, H., Yıldız, M., Zengin, D.M. et al. Intramolecular hydrogen bonding and tautomerism in N-(3-pyridil)-2-oxo-1-naphthylidenemethylamine. Journal of Chemical Crystallography 31, 211–216 (2001). https://doi.org/10.1023/A:1014347216821
Issue Date:
DOI: https://doi.org/10.1023/A:1014347216821