Abstract
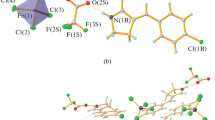
1-Methylpiperazine was employed to crystallize with 2,4-dihydroxybenzoic acid and 1,8-naphthalene acid, affording two multi-component hydrogen-bonding salts [(C5H14N2)2+·(C7H5O4)2 −·H2O](1) and [(C5H14N2)2+(C12H6O4)2−·2H2O](2). These two forms of salts are both monoclinic systems with space group P21/c(14). The lattice parameters of salts 1 and 2 are a=1.32666(10) nm, b=0.90527(7) nm, c=1.67107(13) nm, β=103.125(1)° and a=1.4950(2) nm, b=0.75242(15) nm, c=1.6563(3) nm, β=92.834(2)°, respectively. Expected classical hydrogen bonds N-H⋯O and O-H⋯O appear in the chargetransfer salts, and asymmetric units of these two forms both contain water molecules which play a significant role in building novel supramolecular architectures. Robust hydrogen-bond interactions between 1-methylpiperazine and aromatic acid provide sufficient driving force to direct the two crystals to three-dimensional structures. Weak interactions C-H⋯O emerging in salts 1 and 2 further enhance their crystal structures. As a consequence, hydrogen-bonding interactions in these compounds afford diverse 3D net supramolecular architectures. Thermal stability of these compounds was investigated by thermogravimetric analysis(TGA).
Similar content being viewed by others
References
Khan M., Enkelmann V., Brunklaus G., J. Am. Chem. Soc., 2010, 132, 5254
Wang L., Zhao L., Xu L. Y., Chen R. X., Yang Y., CrystEngComm, 2012, 14, 6998
Wang L., Zhao L., Liu M., Chen R. X., Yang Y., Gu Y. Y., Sci. China Chem., 2012, 55, 2115
Wang L., Zhao L., Xue R. Y., Lu X. F., Wen Y. H., Yang Y., Sci. China Chem., 2012, 55, 2515
Du M., Zhang Z. H., Zhao X. J., Cryst. Growth Des., 2005, 5, 1247
Desiraju G. R., Chem. Commun., 1997, 16, 1475
Desiraju G. R., Angew. Chem. Int. Ed., 2007, 46, 8342
Brammer L., Chem. Soc. Rev., 2004, 33, 476
Wang L., Xu L. Y., Xue R. F., Lu X. F., Chen R. X., Tao X. T., Sci. China Chem., 2012, 55, 138
Wang L., Zhao L., Hu Y. J., Wang W. Q., Chen R. X., Yang Y., CrystEngComm, 2013, 15, 2835
Wang L., Hu Y. J., Wang W. Q., Liu F. Q., Huang K. K., CrystEngComm, 2014, 16, 4142
Hulme A. T., Price S. L., Tocher D. A., J. Am. Chem. Soc., 2005, 127, 1116
Kitagawa S., Kitaura R., Noro S., Angew. Chem. Int. Ed., 2004, 43, 2334
Ferey G., Chem. Soc. Rev., 2008, 37, 191
Trivedi D. R., Ballabh A., Dastidar P., CrystEngComm, 2003, 5, 358
Ward M. D., Chem. Commun, 2005, 48, 5838
Yuge T., Miyata M., Tohnai N., Cryst. Growth Des., 2006, 6, 1271
AakerÖy C. B., Beatty A. M., Helfrich B. A., J. Am. Chem. Soc., 2002, 124, 14425
Vishweshwar P., Nangia A., Lynch V. M., J. Org. Chem., 2002, 67, 556
Vishweshwar P., Nangia A., Lynch V. M., Cryst. Growth Des., 2003, 3, 783
Wang S. H., Hu H. Z., Chen C., Ma R. N., Zhang N., Chem. J. Chinese Universities, 2014, 35(10), 2055
Guo M. L., Acta Cryst. C., 2004, 60, o690
SAINT Software Reference Manual, Bruker AXS, Madison, WI, 1998
Sheldrick G. M., SHELXTL NT Version 5.1, Program for Solution and Refinement of Crystal Structures, University of Göttingen, Göttingen, 1997
Chen Z. Y., Peng M. X., Chin. J. Chem., 2008, 26, 1555
Sarma B., Nath N. K., Bhogala B. R., Nangia A., Cryst. Growth Des., 2009, 9, 1546
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(Nos.51372125, 21203106), the Fund of the State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, China(No.2013-34), and the Scientific and Technical Development Project of Qingdao City, China(No.13-1-4-184-jch).
Rights and permissions
About this article
Cite this article
Yang, Y., Xu, W., Hu, Y. et al. Multi-component hydrogen-bonding organic salts formed from 1-methylpiperazine with aromatic carboxylic acids: Synthons cooperation and crystal structures. Chem. Res. Chin. Univ. 31, 9–15 (2015). https://doi.org/10.1007/s40242-015-4304-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-015-4304-2