Abstract
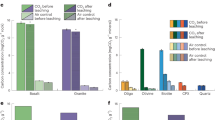
The mechanical activation (MA) of diopside in a controlled CO2 atmosphere was studied using different grinding facilities. The results demonstrate that diopside may sorb CO2 by two mechanisms, depending on the nature of the MA process. If grinding is not accompanied by structure breakdown, the sorption process is similar to that reported for metal oxides, and the adsorbate consists of undistorted CO3 2- groups. If the MA process leads to diopside amorphization, CO2 is sorbed in the form of distorted carbonate groups, and the IR spectrum contains a split absorption band (1433 and 1522 cm–1) similar to the CO3 2- band in the spectra of carbonate-containing silicate glasses. After ball milling in an AL-1000 mechanical activator in CO2 for 580 min, diopside contains up to 15 wt % CO2 . Subsequent heating ensures partial or complete removal of the carbonate. Acid treatment of diopside after MA in CO2 leads to decomposition of the carbonate and almost complete leaching of the Ca and Mg cations.
Similar content being viewed by others
REFERENCES
Avvakumov, E.G., Mekhanicheskie metody aktivatsii khimicheskikh protsessov (Mechanical Activation of Chemical Processes), Novosibirsk: Nauka, 1986.
Boldyrev, V.V., Eksperimental'nye metody v mekhanokhimii tverdykh neorganicheskikh veshchestv (Experimental Methods in the Mechanochemistry of Inorganic Substances), Novosibirsk: Nauka, 1983.
Heinicke, G., Tribochemistry, Munich: Carl Hanser, 1984.
Khodakov, G.S., Sorption Mechanochemistry of Solid Inorganic Materials, Kolloidn. Zh., 1994, vol. 56, no. 1, pp. 113-128.
Bystrikov, A.V., Berestetskaya, I.V., Streletskii, A.N., and Butyagin, P.Yu., Mechanochemistry of the Quartz Surface: I. Products of Reaction with Hydrogen, Kinet. Katal., 1980, vol. 21, no. 3, pp. 765-769.
Streletskii, A.N. and Butyagin, P.Yu., Mechanochemistry of the Quartz Surface: II. Role of Friction, Kinet. Katal., 1980, vol. 21, no. 3, pp. 770-775.
Bystrikov, A.V., Streletskii, A.N., and Butyagin, P.Yu., Mechanochemistry of the Quartz Surface: III. Active Centers in Reaction with Hydrogen, Kinet. Katal., 1980, vol. 21, no. 4, pp. 1013-1018.
Berestetskaya, I.V., Bystrikov, A.V., Streletskii, A.N., and Butyagin, P.Yu., Mechanochemistry of the Quartz Surface: IV. Reaction with Oxygen, Kinet. Katal., 1980, vol. 21, no. 4, pp. 1019-1022.
Bystrikov, A.V., Streletskii, A.N., and Butyagin, P.Yu., Mechanochemistry of the Quartz Surface: V. Oxidation of Carbon Monoxide, Kinet. Katal., 1980, vol. 21, no. 5, pp. 1148-1153.
Kolbanev, I.V., Berestetskaya, I.V., and Butyagin, P.Yu., Mechanochemistry of the Quartz Surface: VI. Properties of the Peroxide Species ?SiÐOÐSi?, Kinet. Katal., 1980, vol. 21, no. 5, pp. 1154-1158.
Ikorskii, S.V. and Evetskaya, E.A., On CO2 Sorption during Gas Elimination from Rocks and Minerals in Vacuum Mills, Geokhimiya, 1975, no. 11, pp. 1712-1719.
Barker, C. and Torkelson, B.E., Gas Adsorption on Crushed Quartz and Basalt, Geochim. Cosmochim. Acta, 1975, vol. 39, no. 2, pp. 212-218.
Kalinkina, E.V., K alinkin, A.M., Forsling, W., and Makarov, V.N., Sorption of Atmospheric Carbon Dioxide and Structural Changes of Ca and Mg Silicate Minerals during Grinding: I. Diopside, Int. J. Miner. Process., 2001, vol. 61, no. 4, pp. 273-288.
Kalinkina, E.V., Kalinkin, A.M., Forsling, W., and Makarov, V.N., Sorption of Atmospheric Carbon Dioxide and Structural Changes of Ca and Mg Silicate Minerals during Grinding: II. Enstatite, Akermanite, and Wollastonite, Int. J. Miner. Process., 2001, vol. 61, no. 4, pp. 289-299.
Rutstein, M.S. and White, W.B., Vibrational Spectra of High-Calcium Pyroxenes and Pyroxenoids, Am. Mineral., 1971, vol. 56, no. 5/6, pp. 877-887.
Fine, G. and Stolper, E., Dissolved Carbon Dioxide in Basaltic Glasses: Concentrations and Speciation, Earth Planet. Sci. Lett., 1985/86, vol. 76, no. 3/4, pp. 263-278.
Sato, M. and Matsuda, S., Structure of Vaterite and Infrared Spectra, Z. Kristallogr., 1969, vol. 129, pp. 405-410.
Little, L.H., Infrared Spectra of Adsorbed Molecules, London: Academic, 1966. Translated under the title Infrakrasnye spektry adsorbirovannykh molekul, Moscow: Mir, 1969.
Butyagin, P.Yu. and Berestetskaya, I.V., Gas Adsorption on the Friction Surface of MgO, Kinet. Katal., 1983, vol. 24, no. 2, pp. 436-440.
Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, New York: Wiley, 1986.Translated under the title IK-spektry i spektry KR neorganicheskikh i koordinatsionnykh soedinenii, Moscow: Mir, 1991.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kalinkin, A.M., Politov, A.A., Boldyrev, V.V. et al. Mechanical Activation of Diopside in CO2. Inorganic Materials 38, 163–167 (2002). https://doi.org/10.1023/A:1014021312359
Issue Date:
DOI: https://doi.org/10.1023/A:1014021312359