Abstract
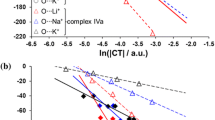
The role of enthalpy, entropy contributions to the shift of complex formation equilibria inwater-organic solvents was studied. The formation of both ammonia and carboxylate complexes of d-metalions was found to be presumably enthalpy-controlled. The role of entropy changes increases in binary solvents with a high level of supramolecular organization, and also in the case of formation of complexes of the highest orders, when the coordination of ligands is accompanied either by a complete displacement of solvent molecules from the inner coordination sphere or by a change in the complex structure. Thus found regularities can be applied for the prediction of heat effects of complex formation in water-organic solvents. In the fist communication [1] of this series we have considered the effects of the nature and composition of water-organic solvents on the stability of ammonia and carboxylate complexes of d-metal ions. This work is based on the data of our recent thermochemical works [2-22] and is dedicated to the study of the role of enthalpy and entropy contributions to the shift of the complex formation equilibria in water-organic solvents.
Similar content being viewed by others
REFERENCES
Sharnin, V.A., Zh. Obshch. Khim., 1999, vol. 69, no. 9, pp. 1421–1429.
Krestov, G.A., Ed., Kompleksoobrazovanie v nevodnykh rastvorakh (Complex Formation in Nonaqueous Solutions), Moscow: Nauka, 1989.
Sharnin, V.A., Shormanov, V.A., and Krestov, G.A., Izv. Vyssh. Uchebn. Zaved., Ser. Khim. Khim. Tekhnol., 1978, vol. 21, no. 6, pp. 831–836.
Sharnin, V.A., Shormanov, V.A., Markov, V.N., and Krestov, G.A., Koord. Khim., 1985, vol. 11, no. 6, pp. 778–783.
Markov, V.N., Sharnin, V.A., and Shormanov, V.A., Zh. Fiz. Khim., 1990, vol. 64, no. 12, pp. 3267–3272.
Nishchenkov, A.V., Sharnin, V.A., Shormanov, V.A., and Krestov, G.A., Koord. Khim., 1991, vol. 17, no. 4, pp.496–500.
Markov, V.N., Sharnin, V.A., Shormanov, V.A., Krestov, G.A., and Isakova, O.A., Koord. Khim., 1991, vol. 17, no. 12, pp. 1704–1707.
Mikheev, S.V., Nischenkov, A.V., Sharnin, V.A., and Shormanov, V.A., Zh. Fiz. Khim., 1992, vol. 66, no. 2, pp. 561–564.
Markov, V.N., Sharnin, V.A., Shormanov, V.A., Krestov, G.A., and Gzheidzyak, A., Koord. Khim., 1992, vol. 18, no. 12, pp. 1219–1223.
Sharnin, V.A., Zh. Obshch. Khim., 1994, vol. 64, no. 11, pp. 1914–1919.
Mikheev, S.V., Sharnin, V.A., and Shormanov, V.A., J. Thermal Anal., 1995, vol. 45, pp. 715–720.
Sharnin, V.A., J. Thermal Anal., 1995, vol. 45, pp. 721–728.
Mikheev, S.V., Sharnin, V.A., Shormanov, V.A., and Krestov, G.A., Zh. Fiz. Khim., 1996, vol. 70, no. 1, pp. 13–16.
Sharnin, V.A., Koord. Khim., 1996, vol. 22, no. 5, pp. 418–421.
Ledenkov, S.F., Shormanov, V.A., and Sharnin, V.A., Zh. Fiz. Khim., 1996, vol. 70, no. 10, pp. 1768–1771.
Shormanov, V.A., Sharnin, V.A., and Ledenkov, S.F., Zh. Neorg. Khim., 1996, vol. 41, no. 5, pp. 877–880.
Shormanov, V.A., Sharnin, V.A., and Ledenkov, S.F., Zh. Fiz. Khim., 1996, vol. 70, no. 8, pp. 1521–1524.
Mikheev, S.V., Sharnin, V.A., and Shormanov, V.A., Zh. Fiz. Khim., 1997, vol. 71, no. 1, pp. 75–77.
Dostizheniya i problemy teorii solvatatsii: strukturnotermodinamicheskie teorii (Progress and Problems of the Theory of Solvation: Structural Thermodynamic Theories), Moscow: Nauka, 1998.
Mikheev, S.V., Sharnin, V.A., and Shormanov, V.A., Zh. Fiz. Khim., 1998, vol. 72, no. 8, pp. 1523–1525.
Ledenkov, S.F., Sharnin, V.A., and Shormanov, V.A., Zh. Neorg. Khim., 1998, vol. 43, no. 2, pp. 344–347.
Sharnin, V.A., Doctorate (Chem.) Disseratation, Ivanovo, 1997.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sharnin, V.A. Regularities in the Influence of Water-Organic Solvents on the Thermodynamics of Complex Formation: II.1 Changes in Enthalpy and Entropy of Formation of Ammonia and Carboxylate Complexes. Russian Journal of General Chemistry 71, 1373–1379 (2001). https://doi.org/10.1023/A:1013949801938
Issue Date:
DOI: https://doi.org/10.1023/A:1013949801938