Abstract
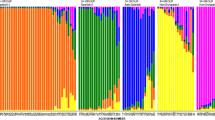
Genetic diversity was estimated among 42 U.S. PlantIntroduction (PI) accessions of the genusCitrullus (of these, 34 PIs are reported tohave disease resistance), and 5 watermelon cultivars, using 30RAPD primers. These primers produced 662 RAPD markers that could berated with high confidence. Based on these markers, geneticsimilarity coefficients were calculated and a dendrogram wasconstructed using the unweighted pair-group method witharithmetic average (UPGMA). The analysis delineated threemajor clusters. The first cluster consisted of a group of fivewatermelon cultivars, a group of C.lanatus var. lanatusaccessions, and a group of C.lanatus var. lanatusaccessions that contained some C.lanatus var. citroidesgenes. The second cluster consisted of the C.lanatus var. citroidesaccessions, while the third cluster consisted of theC. colocynthis accessions.The two C. lanatus clustersdifferentiated from each other and from the C.colocynthis cluster at the level of 58.8%and 38.9% genetic similarity, respectively. Assessment ofgenetic diversity among accessions that have been reported to havedisease resistance indicated that resistance to either anthracnose,downy mildew, powdery mildew, or watermelon mosaic virus is foundamong all major groups of Citrullus PIs.Additionally, resistance to gummy stem blight or Fusarium wilt mayexist among C. lanatus var.citroides PIs. This study demonstrates thatmolecular markers can be useful in assessing genetic diversity, andin sorting Citrullus PIs into phylogeneticgroups prior to their evaluation for disease or pestresistance.
Similar content being viewed by others
References
Biles C.L., Martyn R.D. and Wilson H.D. 1989. Isozymes and general proteins from various watermelon cultivars and tissue types. HortScience 24: 810–812.
Boyhan G.E., Norton J.D., Abrahams B.R. and Wen N.H. 1994. A new source of resistance to anthracnose (Race 2) in watermelon. HortScience 29: 111–112.
Burkill H.M. 1985. The Useful Plants of West Tropical Africa. Royal Botanic Gardens, Kew, 2nd edn. 1.
Dane F., Hawkins L.K. and Norton J.D. 1998. New resistance to race 2 of Fusarium oxysporum f. sp. niveum in watermelon. Cucurbit Genet. Coop. Report 21: 37–39.
De Winter B. 1990. A new species of Citrullus (Benincaseae) from the Namib desert, Namibia. Bothalia 20: 209–211.
Gillaspie A.G. Jr and Wright J.M. 1993. Evaluation of Citrullus sp. germ plasm for resistance to watermelon Mosaic Virus 2. Plant Dis. 77: 352–354.
Hashizume T., Sato T. and Hirai M. 1993. Determination of genetic purity of hybrid seed in watermelon (Citrullus lanatus) and tomato (Lycopersicon esculentum) using random amplified polymorphic DNA (RAPD). Jpn. J. Breed. 43: 367–375.
Hashizume T., Skimamoto I., Harushima Y., Yui M., Sato T., Imai T. et al. 1996. Construction of a linkage map for watermelon [Citrullus lanatus (Thunb.) Matsum & Nakai] using random amplified polymorphic DNA (RAPD). Euphytica 90: 265–273.
Jarret R.L., Merrick L.C., Holms T., Evans J. and Aradhya M.K. 1997. Simple sequence repeats in watermelon [Citrullus lanatus (Thunb.) Matsum. & Nakai]. Genome 40: 433–441.
Jenkins S.F., Winstead N.N. and McCombs C.L. 1964. Pathogenic comparison of three new and four previously described races of Glomerella angulata var. orbiculare. Plant. Dis. Rptr. 48: 619–623.
Katzir N., Danin-Poleg Y., Tzuri G., Karchi Z., Lavi U. and Creagan P.B. 1996. Length polymorphism and homologies of microsatellites in several Cucurbitaceae species. Theor. Appl. Genet. 93: 1282–1290.
Lee S.J., Shin J.S., Park K.W. and Hong Y.P. 1996. Detection of genetic diversity using RAPD-PCR and sugar analysis in water melon [Citrullus lanatus (Thunb.) Mansf.] germplasm. Theor. Appl. Genet. 92: 719–725.
Levi A., Rowland L.J. and Hartung J.S. 1993. Production of reliable randomly amplified polymorphic DNA (RAPD) markers from DNA of woody plants. HortScience 28: 1188–1190.
Levi A. and Thomas C.E. 1999. An improved procedure for isolation of high quality DNA from watermelon and melon leaves. Cucurbit Genet. Coop. Report 22: 41–42.
Martyn R.D. and Netzer D. 1991. Resistance to races 0, 1 and 2 of Fusarium wilt of watermelon in Citrullus sp. PI-296341-FR. HortScience 26: 429–432.
Meeuse A.D. 1962. The Cucurbitaceae of Southern Africa. Bothalia 8: 1–111.
Navot N. and Zamir D. 1987. Isozyme and seed protein phylogeny of the genusCitrullus (Cucurbitaceae). Plant Syst. Evol. 156: 61–67.
Navot N., Sarfatti M. and Zamir D. 1990. Linkage relationships of genes affecting bitterness and flesh color in watermelon. J. Hered. 81: 162–165.
Nei M. and Li W. 1979. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 76: 5269–5273.
Netzer D. and Martyn R.D. 1989. PI 296341, a source of resistance in watermelon to race 2 of Fusarium oxysporum f. sp. niveum. Plant Disease 73: 518.
Rohlf F.J. 1993. NTSYS-PC Numerical Taxonomy and Multi-variate Analysis System. Exter Publishing, Ltd., Setauket, New York, Version 2.00.
Sambrook J., Fritsch E.F. and Maniatis T. 1989. Molecular Cloning. A Laboratory Manual. 2nd edn. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
Simmons A.M. and Levi A. 2000. Evaluation of watermelon germplasm for resistance to Bemisia. Entomological Society of America Annual Meeting. Abstract No. 15611.
Sowell G. Jr 1975. An additional source of resistance to gummy stem blight in watermelon. Plant Dis. Reptr. 59: 413–415.
Sowell G. Jr and Pointer G.R. 1962. Gummy stem blight resistance of introduced watermelons. Plant Dis. Reptr. 46: 883–885.
Sowell G. Jr, Rhodes B.B. and Norton J.D. 1980. New Sources of resistance to watermelon anthracnose. J. Amer. Soc. Hort. Sci. 105: 197–199.
Whitaker T.W. and Bemis W.B. 1976. Cucurbits. In: Simmonds N.W (ed.), Evolution of crop plants. Longman, London, pp. 64–69.
Zamir D., Navot N. and Rudich J. 1984. Enzyme polymorphism in Citrullus lanatus andC. colocynthis in Israel and Sinai. Plant Syst. Evol. 146: 163–137.
Zhang X.P., Rhodes B.B. and Skorupska H.S. 1994. RAPD molecular markers in watermelon. Cucurbit Genet. Coop. Report 17: 116–119.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Levi, A., Thomas, C.E., Keinath, A.P. et al. Genetic diversity among watermelon (Citrullus lanatus and Citrullus colocynthis) accessions. Genetic Resources and Crop Evolution 48, 559–566 (2001). https://doi.org/10.1023/A:1013888418442
Issue Date:
DOI: https://doi.org/10.1023/A:1013888418442