Abstract
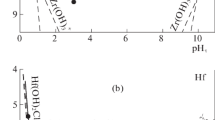
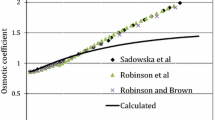
The solubility of HfO2(am) was determined at different equilibration periods from the over- and undersaturation directions, in very acidic to basic solutions (0.1 m HCl to 3.2 m NaOH), and in NaCl solutions ranging in concentrations from very dilute to as high as 5.59 m and in a \({\text{p}}C_{{\text{H}} + }\) range from 1 to 4 to obtain reliable thermodynamic data for the Hf4+–Cl−–Na+–H+–OH−–H2O system. The studies indicate that equilibrium is reached rapidly (<5 days) and that HfO2(am) solubility shows amphoteric behavior. The solubility data obtained in this study, along with the data reported in the literature, at NaOH molalities as high as 21.7 m were interpreted using the ion-interaction model of Pitzer. The log K 0 for the solubility of HfO2(am) [HfO2(am) + 2H2O ⇆ Hf4+ + 4OH−] was determined to be −55.1 ± 0.7. The log K 0 values for the formation of HfOH3+, Hf(OH)0 4, Hf(OH)5 −, and Hf(OH)6 2− according to the reaction (Hf4+ + xOH− ⇆ Hf(OH)4−x x) were determined to be 13.8, <44.8, 49.7 ± 0.2, and 51.2 ± 0.2, respectively. The thermodynamic model developed in this study is valid for a wide range of conditions (as high as 0.1 m HCl, 21.7 m NaOH, and 5.59 m NaCl). The binary ion-interaction parameters for Hf4+–Cl−, HfOH3+–Cl−, and Hf(OH)2− 6–Na+ were determined in this study to accurately define the observed solubility behavior of hafnium in various systems.
Similar content being viewed by others
REFERENCES
H. T. Weger, D. Rai, N. J. Hess, and B. P. McGrail, Solubility and Aqueous-Phase Reactions of Gadolinium in the K +–Na+i–CO 2-3 –OH–H2O System PNNL-11864 (Pacific Northwest National Laboratory, Richland, Washington, 1998).
E. M. Larsen and A. M. Gammill, J. Amer. Chem. Soc. 72, 3615 (1950).
E. M. Larsen and P. Wang, J. Amer. Chem. Soc. 76, 6223 (1954).
D. B. Copley and S. Y. Tyree, Jr., Inorg. Chem. 7/7, 1472 (1968).
J. S. Johnson, K. A. Kraus, and R. W. Holmberg, J. Amer. Chem. Soc. 78, 26 (1956).
E. H. Huffman, G. M. Iddings, and R. C. Lilly, J. Amer. Chem. Soc. 73, 4474 (1951).
J. S. Johnson and K. A. Kraus, J. Amer. Chem. Soc. 78, 3937 (1956).
A. J. Zielen and R. E. Connick, J. Amer. Chem. Soc. 78, 5785 (1956).
E. N. Lebedeva, S. S. Korovin, and A. M. Rozen, Russ. J. Inorg. Chem. 9/7, 944 (1964).
C. F. Baes, Jr. and R. F. Mesmer, The Hydrolysis of Cations (Wiley, New York, 1976).
B. Noren, Acta Chem. Scandi. 27, 1369 (1973).
G. D. M. Cerefìce, K. Noyes, and K. Czerwinski, Mat. Res. Soc. Symp. Proc. 556, 1025 (1999).
P. N. Kovalenko and K. N. Bagdasarov, Russ. J. Inorg. Chem. 7/18, 913 (1962).
V. P. Vasil'ev, A. I. Lytkin, and N. V. Chernyavskaya, J. Thermal Analy. Calorimetry 55, 1003 (1999).
L. G. M. Baas Becking, I. R. Kaplan, and D. A. Moore, J. Geol. 68, 243 (1960).
V. M. Peshkova and P. Pen, Vestn. Mosk. Univ., Ser. II Khim. 18/1, 40 (1963).
R. G. Deshpande, P. K. Khopkar, C. L. Rao, and H. D. Sharma, J. Inorg. Nucl. Chem. 27, 2171 (1965).
J. Hála and D. Pohanková, J. Inorg. Nucl. Chem. 29, 2983 (1967).
I. N. Marov and D. I. Ryabchikov, Zh. Neorg. Khim. 7, 1036 (1962).
A. N. Ermakov, L. N. Marov, and G. A. Evtikova, Russ. J. Inorg. Chem. 12/12, 1784 (1967).
D. Rai, A. R. Felmy, S. P. Juracich, and L. Rao, Estimating the Hydrogen Ion Concentration in Concentrated NaCl and Na 2SO4 Electrolytes, SAND94-1949 (Sandia National Laboratories, Albuquerque, New Mexico, 1995).
I. A. Sheka and Ts. V. Pevzner, Russ. J. Inorg. Chem. 5/10, 1119 (1960).
D. Rai, N. J. Hess, A. R. Felmy, D. A. Moore, M. Yui, and P. Vitorge, Radiochim. Acta 86, 89 (1999).
R. Ruh and P. W. R. Corfield, Amer. Ceramic Soc. 53, 119 (1970).
A. L. Ankudinov, Ph.D. Thesis, University of Washington, Washington, 1996.
J. J. Rehr, J. Leon de Mustre, S. I. Zabinsky, and R. C. Albers, J. Amer. Chem. Soc. 113, 5135 (1991).
K. S. Pitzer and G. Mayorga, J. Phys. Chem. 77, 2300 (1973).
K. S. Pitzer, in Activity Coefficients in Electrolyte Solutions, K. S. Pitzer, ed. (CRC Press, Boca Raton, FL, 1991) Chapt. 3, pp. 75–153.
A. R. Felmy and J. H. Weare, Geochim. Cosmochim. Acta 50, 2771 (1986).
A. R. Felmy, D. Rai, J. A. Schramke, and J. L. Ryan, Radiochim. Acta 48, 29 (1989).
S. M. Sterner, A. R. Felmy, J. R. Rustad, and K. S. Pitzer, Thermodynamic Analysis of Aqueous Solutions Using INSIGHT, PNWD-SA-4436 (Pacific Northwest National Laboratory, Richland, Washington, 1997).
A. R. Felmy, D. Rai, S. M. Sterner, M. J. Mason, N. J. Hess, and S. D. Conradson, J. Solution Chem. 26, 233 (1997).
D. Rai, A. R. Felmy, S. M. Sterner, D. A. Moore, M. J. Mason, and C. F. Novak, Radiochim. Acta 79, 239 (1997).
D. Rai, A. R. Felmy, N. J. Hess, D. A. Moore, and M. Yui, Radiochim. Acta 82, 17 (1998).
D. Rai, A. R. Felmy, N. J. Hess, D. A. Moore, and M. Yui, Radiochim. Acta 84, 159 (1999).
D. Rai, A. R. Felmy, and J. L. Ryan, Inorg. Chem. 29, 260 (1990).
I. J. Grenthe, R. J. Fuger, M. Konigs, R. J. Lemire, A. B. Muller, C. Nguyen-Trung, and H. Wannter, Chemical Thermodynamics of Uranium, Vol. 1 (Elsevier, New York, 1992).
C. E. Harvie, N. Moller, and J. H. Weare, Geochim. Cosmochim. Acta 48, 723 (1984).
D. Rai, A. R. Felmy, and R. W. Szelmeczka, J. Solution Chem. 20/4, 375 (1991).
R. A. Day, Jr., R. N. Wilhite, and F. D. Hamilton, J. Amer. Chem. Soc. 77, 3180 (1955).
F. T. Bunus, J. Inorg. Nucl. Chem. 36, 917 (1974).
J. Sobkowski, J. Inorg. Nucl. Chem. 23, 81 (1961).
R. C. Weast, Handbook of Chemistry and Physics (CRC Press, Cleveland, OH, 1972–1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rai, D., Xia, Y., Hess, N.J. et al. Hydroxo and Chloro Complexes/Ion Interactions of Hf4+ and the Solubility Product of HfO2(am). Journal of Solution Chemistry 30, 949–967 (2001). https://doi.org/10.1023/A:1013337925441
Issue Date:
DOI: https://doi.org/10.1023/A:1013337925441