Abstract
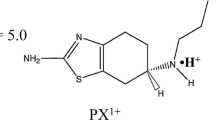
Purpose. The objectives of this work were 1) to establish the feasibility of the transdermal iontophoretic delivery of ropinirole hydrochloride; 2) to investigate the possibility of delivering therapeutic doses of this drug; and 3) to determine the key factors that control ropinirole electrotransport.
Methods. A series of in vitro transdermal iontophoretic experiments were instituted to study the effects of drug concentration, co-ion concentration, intensity of current, and application time on ropinirole flux. The convective contribution to ropinirole electrotransport was evaluated by following the transport of the electroosmotic marker mannitol.
Results. Ropinirole flux decreased dramatically in the presence of competing ions. This effect was observed even when the molar fraction of the two competing cations was kept constant. Anodal flux of mannitol decreased with drug concentration, indicating a possible alteration of the skin permselectivity. In the absence of competing co-ions, ropinirole transport number reached a maximum value (8-13%). In these conditions, the main factor controlling drug delivery was the intensity of current applied.
Conclusions. Transdermal iontophoresis allowed the delivery of therapeutic doses of ropinirole. The dose administered and the input rate were controlled by the judicious choice of the key delivery factors here described.
Similar content being viewed by others
REFERENCES
I. F. Tulloch. Pharmacologic profile of Ropinirole. Neurology 49:S58-S62 (1997).
M. J. Vidalilhet, A. M. Bonnet, S. Belal, B. Dubois, C. Marle, and Y. Agid. Ropinirole without levodopa in Parkinson's disease. Lancet 336:316-317 (1990).
A. J. Matheson and C. M. Spencer. Ropinirole. A review of its use in the management of Parkinson's disease. Drugs 60: 115-137 (2000).
C. H. Adler, K. D. Sethi, R.A. Hauser, T. L. Davis, J. P. Hammarstad, J. Bertoni, R. L. Taylor, J. Sanchez-Ramos, and C. F. O'Brien. Ropinirole for the treatment of early Parkinson's disease. Neurology 49:393-399 (1997).
A. E. Scharg, D. J. Brooks, E. Brunt, D. Fuell, A. Korczyn, W. Poewe, N. P. Quinn, O. Rascol, and F. Stocchi. The safety of Ropinirole, a selective nonergoline dopamine agonist, in patients with Parkinson's disease. Neuropharmacology 21:169-175 (1998).
B. H. Sage. Iontophoresis. In E. W. Smith, H. I. Maibach, (eds), Percutaneous Penetration Enhancers. CRC Press, Boca Raton, Florida 1995 pp 351-368.
R. R. Burnette and B. Ongipattanakul. Characterization of the permselective properties of excised human skin during iontophoresis. J. Pharm. Sci. 76:765-773 (1987).
P. G. Green, R. S. Hinz, C. Cullander, G. Yamane, and R. H. Guy. Iontophoretic delivery of aminoacids and aminoacid derivatives across the skin in vitro. Pharm. Res. 8:1113-1120 (1991).
P. W. Atkins. Physical Chemistry. Oxford University Press, 1978.
W. J. Moore. Physical Chemistry. Prentice-Hall Inc, London, 1972.
D. Marro, M. B. Delgado-Charro, Y. N. Kalia, and R. H. Guy. Iontophoretic transport mechanisms: Effect of background electrolyte and competing ions. Proceed. Intl Symp. Control. Release Bioact. Mater. 26:94-95 (1999).
G. B. Kasting and J. C. Keister. Application of electrodiffusion theory for a homogeneous membrane to ion iontophoretic transport trough skin. J. Control. Release 8:195-210 (1989).
R. V. Padmanabhan, J. B. Phipps, G. A. Lattin, and R. J. Sawchuk. In vitro and in vivo evaluation of transdermal iontophoretic delivery of hydromorphone. J. Control. Release 11:123-135 (1990).
L. Wearly, J. C. Liu, and Y. W. Chien. Iontophoresis facilitated transdermal delivery of verapamil II. Factors affecting the skin permeability. J. Control. Release 9:231-242 (1989).
S. Thysman, C. Tasset, and V. Préat. Transdermal iontophoresis of fentanyl: delivery and mechanistic analysis. Int. J. Pharm. 101:105-113 (1994).
L. L. Miller and G. A. Smith. Iontophoretic transport of acetate and carboxylate ions through hairless mouse skin: cation exchange membrane model. Int. J. Pharm. 49:15-22 (1989).
M. B. Delgado-Charro and R. H. Guy. Iontophoretic delivery of nafarelin across the skin. Int. J. Pharm. 117:165-172 (1995).
A. J. Hoogstraate, V. Srinivasan, S. M. Sims, and W. I. Higuchi. Iontophoretic enhancement of peptides: Behavior of leuprolide versus model permeants. J. Control. Release 31:41-47 (1994).
J. Hirvonen and R. H. Guy. Iontophoretic delivery across the skin: electroosmosis and its modulation by drug substances. J. Control. Release 14:1258-1262 (1997).
J. Hirvonen, Y. N. Kalia, and R. H. Guy. Transdermal delivery of peptides by iontophoresis. Nat. Biotech. 14:1710-1713 (1996).
A. Kim, P. G. Green, G. Rao, and R. H. Guy. Convective solvent flow across the skin during iontophoresis. Pharm. Res. 10:1315-1320 (1993).
P. Santi and R. H. Guy. Reverse iontophoresis—Parameters determining electroosmotic flow: I. pH and ionic strength. J. Control. Release 38:159-165 (1996).
M. P. Pikal and S. Shah. Transport mechanisms in iontophoresis II. Electroosmotic flow and transference number measurements for hairless mouse skin. Pharm. Res. 7:213-223 (1990).
J. B. Phipps and J. R. Gyory. Transdermal ion migration. Adv. Drug Deliv. Rev. 9:137-176 (1992).
D. Marro, M. B. Delgado-Charro, Y. N. Kalia, and R. H. Guy. Optimizing iontophoretic drug delivery: Identification and distribution of the charge-carrying species. Proceed. Intl Symp. Control. Release Bioact. Mater. 27:938-939 (2000).
N. H. Yoshida and M. S. Roberts. Prediction of cathodal iontophoretic transport of various anions across excised skin form different vehicles using conductivity measurements. J. Pharm. Pharmacol. 47:883-890 (1995).
N. H. Yoshida and M. S. Roberts. Role of conductivity in iontophoresis. Part 2. Anodal iontophoretic transport of phenylethylamine and sodium across excised human skin. J. Pharm. Sci. 35:350-344 (1994).
R. C. Weast. Handbook of Chemistry and Physics, 55th Edition. CRC Press, Cleveland, Ohio 1974.
A. Luzardo-Alvarez, M. Rodriguez-Fernandez, J. Blanco-Mendez, R. H. Guy, and M. B. Delgado-Charro. Iontophoretic permselectivity of mammalian skin: characterization of hairless mouse and porcine membrane models. Pharm. Res. 15:984-987 (1998).
M. B. Delgado-Charro and R. H. Guy. Characterization of convective solvent flow during iontophoresis. Pharm. Res. 11:929-935 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luzardo-Alvarez, A., Delgado-Charro, M.B. & Blanco-Méndez, J. Iontophoretic Delivery of Ropinirole Hydrochloride: Effect of Current Density and Vehicle Formulation. Pharm Res 18, 1714–1720 (2001). https://doi.org/10.1023/A:1013322613436
Issue Date:
DOI: https://doi.org/10.1023/A:1013322613436