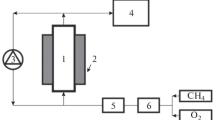
Abstract
Deep oxidation of methane on the granulated Cu—Mn-mixed oxide catalyst and metallic monolith catalysts coated with the same oxide was studied. The experimental kinetic curves for both monolith and granulated catalysts are satisfactorily described by the first-order rate law. The values of activation energies, reaction rate constants, and feed flow rates for the specified conversion almost coincide for both types of the catalysts. The data obtained confirm the possibility of a quantitative comparison of the activities of the granulated and monolith catalysts. The activity of the monoliths is proportional to the concentration of the active component.
Similar content being viewed by others
References
V. D. Meshcheryakov, V. A. Kirilov, and N. A. Kuzin, Teoreticheskie osnovy khimicheskoi tekhnologii [Theoretical Foundations of Chemical Technology], 1999, 33, 561 (in Russian).
L. Löwendahl and J.-E. Otterstedt, Appl. Catal., 1990, 59, 89.
Z. R. Ismagilov, R. A. Shkrabina, D. A. Arendarskii, and N. V. Shikina, Kinet. Katal., 1998, 39, 653 [Kinet. Catal., 1998, 39 (Engl. Transl.)].
E. A. Trusova, M. V. Tsodikov, E. V. Slivinskii, and V. G. Lipovich, Neftekhimiya, 1999, 39, 243 [Petrol. Chem., 1999, 39 (Engl. Transl.)].
L. T. Tsikoza, Z. R. Ismagilov, R. A. Shkrabina, V. A. Sazonov, and N. V. Shikina, Kinet. Katal., 1998, 39, 661 [Kinet. Catal., 1998, 39 (Engl. Transl.)].
A. V. Sagalovich and A. L. Klyachko-Gurvich, Usp. Khim., 1971, 40, 1237 [Russ. Chem. Rev., 1971, 40 (Engl. Transl.)].
E. V. Rebrov, A. V. Simakov, N. N. Sazonova, O. V. Komova, N. A. Kulikovskaya, T. A. Nikoro, and G. B. Barannik, Kinet. Katal., 1998, 39, 716 [Kinet. Catal., 1998, 39 (Engl. Transl.)].
L. A. Isupova, G. A. Alikina, O. I. Snegurenko, V. A. Sadykov, and S. V. Tsybulya, Appl. Catal. B: Environmental, 1999, 21, 171.
M. P. Vorob'eva, A. A. Greish, A. V. Ivanov, and L. M. Kustov, Appl. Catal. A: General, 2000, 199, 257.
S. L. Kiperman, Vvedenie v kinetiku geterogennykh kataliticheskikh reaktsii [Introduction into Kinetics of Heterogeneous Reactions], Nauka, Moscow, 1964, 558 (in Russian).
C. N. Satterfield, Mass Transfer in Heterogeneous Catalysis, M.I.T., Mass., Cambridge, 1970.
S. N. Budar, Usp. Khim., 1974, 43, 320 [Russ. Chem. Bull., 1974, 43 (Engl. Transl.)].
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Slovetskaya, K.I., Greish, A.A., Vorob"eva, M.P. et al. Deep oxidation of methane on granulated and monolith copper—manganese oxide catalysts. Russian Chemical Bulletin 50, 1589–1592 (2001). https://doi.org/10.1023/A:1013078316977
Issue Date:
DOI: https://doi.org/10.1023/A:1013078316977