Abstract
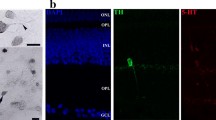
Glutamate and GABA are the major excitatory and inhibitory neurotransmitters in the CNS, including the retina. In the chick retina, GABA is located in horizontal and amacrine cells and in some cells in the ganglion cell layer. It has been shown that glutamate and its agonists, NMDA, kainate, and aspartate, promote the release of GABA from isolated retina and from cultured retinal cells. Dopamine, the major catecholamine in the retina, inhibits the induction of GABA release by NMDA. Two to seven-day-old intact chicken retinas were stimulated with different glutamatergic agonists and the GABA remaining in the tissue was detected by immunohistochemical procedures. The exposure of retinas to 100 μ M NMDA for 30 minutes resulted in 50% reduction in the number of GABA-immunoreactive amacrine cells. Aspartate (100 μ M) treatment also resulted in 60% decrease in the number of GABA-immunoreactive amacrine cells. The number of GABA-immunoreactive horizontal cells was not affected by either NMDA or aspartate. In addition, dopamine reversed by 50% the reduction of the number of GABA-immunoreactive amacrine cells exposed to NMDA or aspartate. Kainate stimulation promoted a 50% reduction in the number of both GABA-immunoreactive amacrine and horizontal cells. Dopamine did not interfere with the kainate effect. While in control and in non-stimulated retinas a continuous and homogeneous immunolabeling was observed throughout the inner plexiform layer, retinas exposed to NMDA, kainate and aspartate displayed only a faint punctate labeling in the inner plexiform layer. It is concluded that, under our experimental conditions, both NMDA and aspartate induce the release of GABA exclusively from amacrine cells, and that the release is modulated by dopamine. On the other hand, kainate stimulates GABA release from both amacrine and horizontal cells with no interference of dopamine.
Similar content being viewed by others
References
Agardh, E., Bruun, A., Ehinger, B., EkstrÖm, P., van Veen, T. & Wu, J. Y. (1987) Gamma-aminobutyric acid-and glutamic acid decarboxylase-immunoreactive neurons in the retina of different vertebrates. Journal of Comparative Neurology 258, 622–630.
Agardh, E., Ehinger, B. & Wu, J. Y. (1987) GABA and GAD-like immunoreactivity in the primate retina. Histochemistry 86, 485–490.
Araki, M., Maeda, T. & Kimura, H. (1983) Dopaminergic cell differentiation in developing chick retina. Brain Research Bulletin 11, 97–102.
Barnstable, C. J. (1993) Glutamate and GABA in retinal circuitry. Current Opinion of Neurobiology 3, 520–525.
Beaulieu, C. & Crevier, C. (1994) Quantitative aspects of the GABA circuitry in the visual cortex of adult rat. Journal of Comparative Neurology 339, 559–572.
Blanco, R. & de la Villa, P. (1999) Ionotropic glutamate receptors in isolated horizontal cells of the rabbit retina. European Journal of Neuroscience 11, 867–873.
Carvalho, A. P., Ferreira, I. L., Carvalho, A. L. & Duarte, C. B. (1995) Glutamate receptor modulation of [3H] GABA release and intracellular calcium in chick retina cells. Annals of the New York Academy of Sciences 757, 439–456.
Castro, N. G., de Mello, M. C. F., de Mello, F. G. & Aracava, Y. (1999) Direct inhibition of the NMDA receptor channel by dopamine and (+)-SKF38393. British Journal of Pharmacology 126, 1847–1855.
Costa, B. L. S. A., de Mello, F. G. & HokoÇ, J. N. (2000) Transporter-mediated GABA release induced by excitatory amino acid agonist is associated with GAD-67, but not GAD-65 immunoreactive cells of the primate retina. Brain Research 863, 132–142.
do Nascimento, J. L. M. & de Mello, F. G. (1985) Induced release of gamma-aminobutyric acid by a carrier-mediated high-affinity uptake of L-glutamate in cultured chick retina cells. Journal of Neurochemistry 45, 1820–1827.
do Nascimento, J. L. M., Kubrusly, R. C. C., Reis, R. A. M., de Mello, M. C. F. & de Mello, F. G. (1998a) Atypical effect of dopamine in modulating the functional inhibition of NMDA receptors of cultured retina cells. European Journal of Pharmacology 343, 103–110.
do Nascimento, J. L. M., Ventura, A. L. M. & Paes de Carvalho, R. (1998b) Veratridine-and glutamateinduced release of [3H]-GABA from cultured chick retina cells: Possible involvement of a GAT-1-like subtype of GABA transporter. Brain Research 798, 217–222.
dos Santos, R. M. & Gardino, P. F. (1998) Differential distribution of a second type of tyrosine hydroxylase immunoreactive amacrine cell in the chick retina. Journal of Neurocytology 27, 33–43.
Duarte, C. B., Ferreira, I. L., Santos, P. F., Oliveira, C. R. & Carvalho, A. P. (1993) Glutamate increases the [Ca2+]i but stimulates Ca2+-independent release of [3H] GABA in cultured chick retina cells. Brain Research 611, 130–138.
Ferreira, I. L., Duarte, C. B., Santos, P. F., Carvalho, C. M. & Carvalho, A. P. (1994) Release of [3H]-GABA evoked by glutamate receptor agonists in cultured avian retina cells: Effect of Ca2+. Brain Research 664, 252–256.
Firth, S. I., Hons, B. S. C. & Morgan, I. G. (1997) Localization of D1 dopamine receptors in the chicken retina. Australian and New Zealand Journal of Ophthalmology 25, S64–S66.
Fischer, A. J., Seltner, R. L. P., Poon, J. & Stell, W. K. (1998) Immunocytochemical characterization of quisqualic acid-and N-Methyl-D-Aspartate-induced excitotoxicity in the retina of chicks. Journal of Comparative Neurology 393, 1–15.
Goebel, D. J., Aurelia, J. L., Tai, Q., Jojich, L. & Poosch, M. S. (1998) Immunocytochemical localization of the NMDA-R2A receptor subunit in the cat retina. Brain Research 808, 141–154.
Hashimoto, A. & Oka, T. (1997) Free D-aspartate and D-serine in the mammalian brain and periphery. Progress in Neurobiology 52, 325–353.
Hof, P. R., Lee, P. Y., Yeung, G., Wang, R. F., Podos, S. M. & Morrison, J. H. (1998) Glutamate receptor subunit GluR2 andNMDAR1immunoreactivity in the retina of macaque monkeys with experimental glaucoma does not identify vulnerable neurons. Experimental Neurology 153, 234–241.
Hofmann, H. D. & MÜckel, V. (1991) Release of gamma-amino (3H) butyric acid from cultured amacrine-like neurons mediated by different excitatory amino acids. Journal of Neurochemistry 56, 923–932.
HokoÇ, J. N., Ventura, A. L. M., Gardino, P. F. & de Mello, F. G. (1990) Developmental immunoreactivity for GABA and GAD in the avian retina: Possible alternative pathway for GABA synthesis. Brain Research 532, 197–202.
Kalloniatis, M. & Fletcher, E. L. (1993) Immunocytochemical localization of the amino acid neurotransmitters in the chicken retina. Journal of Comparative Neurology 336, 174–193.
Kamada, Y., Mizuno, A. & Matsuda, M. (1981) Effect of L-glutamic acid on [14C]GABA release from isolated rat retina. Brain Research 229, 251–255.
Kato, S., Negishi, K. & Teranishi, T. (1984) Embryonic development of monoaminergic neurons in the chick retina. Journal of Comparative Neurology 224, 437–444.
Kubrusly, R. C. C., de Mello, M. C. F. & de Mello, F. G. (1998) Aspartate as a selective NMDA receptor agonist in cultured cells from the avian retina. Neurochemistry International 32, 47–52.
Lee, J. A., Homma, H., Tashiro, K., Iwatsubo, T. & Imai, K. (1999) D-Aspartate localization in the rat pituitary gland and retina. Brain Research 838, 193–199.
LÓpez-Colome, A. M. & Somohano, F. (1986) Effect of selective kainate lesions on the release of glutamate and aspartate from chick retina. Journal of Neuroscience Research 15, 205–216.
Marc, R. E. (1999) Mapping glutamatergic drive in the vertebrate retina with a channel-permeant organic cation. Journal of Comparative Neurology 407, 47–64.
Massey, S. C. & Maguire, G. (1995) The role of glutamate in retinal circuitry. Excitatory amino acids and synaptic transmission. In Excitatory Amino Acids and Synaptic Transmission (edited by Wheal, H. & Thomson, A.) pp. 201–221. San Diego: Academic Press.
Massey, S. C. & Redburn, D. (1987) Transmitter circuits in the vertebrate retina. Progress in Neurobiology 28, 55–96.
MorÁn, J. & Pasantes-Morales, H. (1983) Effects of excitatory amino acids, and of their agonist and antagonists on the release of neurotransmitters from the chick retina. Journal of Neuroscience Research 10, 261–271.
Mosinger, J. L., Yazulla, S. & Studholme, K. M. (1986) GABA-like immunoreactivity in the vertebrate retina: A species comparison. Experimental Eye Research 42, 631–644.
Osborne, N. N., Patel, S., Beaton, D. W. & Neuhoff, V. (1986) GABA neurones in retinas of different species and their postnatal development in situ and in culture in the rabbit retina. Cell Tissue Research 243, 117–123.
Osborne, N. N., Larsen, A. & Barnett, N. L. (1995) Influence of excitatory amino acids and isquemia on rat retinal choline acetyltransferase-containing cells. Investigative Ophthalmology Visual Science 36, 1692–1700.
Rohrer, B. & Stell, W. K. (1995) Localization of putative dopamine D2-like receptors in the chick retina, using in situ hybridization and immunocytochemistry. Brain Research 695, 110–116.
Schwarcz, R. & Coyle, J. T. (1977) Kainic acid: Neurotoxic effects after intraocular injection. Investigative Ophthalmology and Visual Research 16, 141–148.
Schwartz, E. A. (1987) Depolarization without calcium can release gamma-aminobutyric acid from retinal neurons. Science 238, 350–355.
Sun, H. & Crossland, W. J. (2000) Quantitative assessment of localization and colocalization of glutamate, aspartate, glycine and GABA immunoreactivity in the chick retina. Anatomical Record 260, 158–179.
Suzdak, P. D., Frederiksen, K., Andersen, K. E., Sorensen, P., Knutsen, B. J. S. & Nielsen, E. B. (1992) NNC-711 a novel potent and selective γ-aminobutyric acid uptake inhibitor: Pharmacological characterization. European Journal of Pharmacology 223, 189–198.
Tachibana, M. & Kaneko, A. (1988) Retinal bipolar cells receive negative feedback input from GABAergic amacrine cells. Visual Neuroscience 1, 297–305.
Tapia, R. & Arias, C. (1982) Selective stimulation of neurotransmitter release from chick retina by kainic and glutamic acids. Journal of Neurochemistry 39, 1169–1178.
Thoreson, W. B. & Witkovsky, P. (1999) Glutamate receptors and circuits in the vertebrate retina. Progress in Retinal and Eye Research 18, 765–810.
Ventura, A. L. & de Mello, F. G. (1990) D1 dopamine receptors in neurite regions of embryonic and differentiated retina are highly coupled to adenylate cyclase in the embryonic but not in the mature tissue. Brain Research 530, 301–308.
Yaqub, A. & Eldred, W. D. (1991) Localization of aspartate-like immunoreactivity in the retina of the turtle (Pseudemys scripta). Journal of Comparative Neurology 312, 584–598.
Yaqub, A. & Eldred, W. D. (1993) Effects of excitatory amino acids on immunocytochemically identified populations of neurons in turtle retina. Journal of Neurocytology 22, 644–662.
Yazulla, S. (1983) Stimulation of GABA release from retinal horizontal cells by potassium and acidic amino acid agonists. Brain Research 275, 61–74.
Yazulla, S. (1986) GABAergic mechanisms in the retina. Progress in Retinal Research 5, 1–12.
Yazulla, S. & Kleinschmidt, J. (1983) Carrier-mediated release of GABA from retinal horizontal cells. Brain Research 263, 63–75.
Yazulla, S., Cunningham, J. O. & Neal, M. (1985) Stimulated release of GABA and glycine from goldfish retina. Brain Research 345, 384–388.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calaza, K.C., de Mello, F.G. & Gardino, P.F. GABA release induced by aspartate-mediated activation of NMDA receptors is modulated by dopamine in a selective subpopulation of amacrine cells. J Neurocytol 30, 181–193 (2001). https://doi.org/10.1023/A:1012764422711
Issue Date:
DOI: https://doi.org/10.1023/A:1012764422711