Abstract
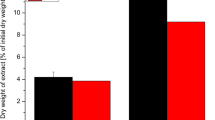
Three steps of the alginate production process were studied at pilot plantlevel. The effect of the amount of calcium chloride used during theprecipitation was measured in terms of filtration time of the precipitatedcalcium alginate. Three different proportions of calcium chloride per gramof alginate were tested. The best proportion used was 2.2 parts ofcalcium chloride per one part of alginate, yielding a filtration rate of 97.9L min-1 on a screen area of 1.32 m2. The method ofadding the solutions and the degree of mixing are discussed as other factorsaffecting the precipitation step. The effect of bleaching the calciumalginate with sodium hypochlorite (5%) was studied. Seven proportions,ranging from 0 to 0.77 mL of sodium hypochlorite per gram of sodiumalginate were tested. The effect of hypochlorite was compared foralginates with three different viscosities. Using alginates with mediumviscosity (300–500 mPa s), the best proportion was 0.4 mL hypochloriteper gram of alginate, yielding an alginate of light cream color with 20%less viscosity than the control. Alginates with lower viscosity showed asmaller loss of viscosity. The effect of pH during conversion of calciumalginate to alginic acid was determined using four combinations of pH,ranging from 2.2 to 1.6, in three acid washings. The extent of conversionwas determined by measuring the percent reduction of the alginate viscosity(RV) in 1% solution before and after adding a sequestrant of calcium. When a pH 1.8 or 1.6 was used for each washing, only two washings werenecessary to produce a RV lower than 40% (maximum recommended). The use of pH 2 required three acid washings to produce the same effect. The pH 2.2 did not remove enough calcium, even with three washings,the RV of the resulting sodium alginate being greater that 40%. Theresults of these experiments provide the information that producers needwhen deciding the best parameters to obtain a product with the desiredcharacteristics.
Similar content being viewed by others
References
Arvizu-Higuera DL, Hernández-Carmona G, Rodríguez-Montesinos YE (1997) Effect of the type of precipitation on the process to obtain sodium alginate: calcium alginate method and alginic acid method. Ciencias Marinas 23(2): 195-207.
Baranov VS, Locev VN, Guernet NA, Kuchumova RP, Kuchumov AM (1967) Method to obtain sodium alginate. USSR Patent 707,561.
Green HC (1936) Process for making alginic acid and product. US Patent 2,036,934.
Hasebe N (1976) Method for treating seaweed with hydrogen peroxide or hydrogen peroxide compound. Australian Patent 17,932.
Haug A (1964) Composition and properties of alginates. Rep. 30. Norwegian Inst. of Seaweed Research. Trondheim, Norway. 123 pp.
Hernández-Carmona G, McHugh DJ, Arvizu-Higuera DL, Rodríguez-Montesinos YE (1999a) Pilot plant scale extraction of alginate from Macrocystis pyrifera. 1. The effect of preextraction treatments on the yield and quality of alginate. J. appl. Phycol. 10(6): 507-513.
Hernández-Carmona G, McHugh DJ, López-Gutiérrez F (1999b) Pilot plant scale extraction of alginate from Macrocystis pyrifera. 2. Studies on extraction conditions and methods of separating the alkaline-insoluble residue. J. appl. Phycol. 11(6): 493-502.
Le Gloahec VCE, Herter JR (1938) Method of treating seaweed. US Patent 2,128,551.
Lukachyov OP, Pochkalov VK (1965) Method to obtain alginate from brown algae. USSR Patent 200,416.
McHugh DJ (1987) Production, properties and uses of alginates. In McHugh DJ (ed.), Production and Utilization of Products from Commercial Seaweeds. FAO. Fish. Tech. Pap. 288: 58-115.
Mizuno H, Iso N, Saito T, Nozawa T (1982) Decoloration of alginic acid products by organic solvents. Bull. Japanese Soc. Scientific Fish. 48(11): 1675.
Schwarzenbach G, Flaschka HA (1969) Complexometric titrations. Barnes & Noble, New York, 490 pp.
Societé Tech. de Recherchers et d'Explotations (1949) French Patent 948,814.
StatSoft, Inc. (1995) 2325 East 13th Street, Tulsa, OK 74104. 1878 pp.
Thornley FC, Walsh MJ (1931) Process of preparing alginic acid and compounds thereof. US Patent 1,814,981.
Zar JH (1984) Biostatistical analysis. Prentice Hall, Inc., Englewood Cliffs, New Jersey, 718 pp.
Zvered DI, Afonin BP, Sendzimerzh AL, Andrievsky YP, Petzakova TN, Andrievskaya VV (1969) Recovery of brown alginates from brown algae. USSR Patent 229,213.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McHugh, D.J., Hernández-Carmona, G., Luz Arvizu-Higuera, D. et al. Pilot plant scale extraction of alginates from Macrocystis pyrifera 3. Precipitation, bleaching and conversion of calcium alginate to alginic acid. Journal of Applied Phycology 13, 471–479 (2001). https://doi.org/10.1023/A:1012532706235
Issue Date:
DOI: https://doi.org/10.1023/A:1012532706235